The Unique Protein-to-Protein Carotenoid Transfer Mechanism
- PMID: 28746851
- PMCID: PMC5529199
- DOI: 10.1016/j.bpj.2017.06.002
The Unique Protein-to-Protein Carotenoid Transfer Mechanism
Abstract
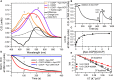
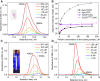
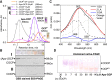
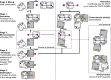
Orange Carotenoid Protein (OCP) is known as an effector and regulator of cyanobacterial photoprotection. This 35 kDa water-soluble protein provides specific environment for blue-green light absorbing keto-carotenoids, which excitation causes dramatic but fully reversible rearrangements of the OCP structure, including carotenoid translocation and separation of C- and N-terminal domains upon transition from the basic orange to photoactivated red OCP form. Although recent studies greatly improved our understanding of the OCP photocycle and interaction with phycobilisomes and the fluorescence recovery protein, the mechanism of OCP assembly remains unclear. Apparently, this process requires targeted delivery and incorporation of a highly hydrophobic carotenoid molecule into the water-soluble apoprotein of OCP. Recently, we introduced, to our knowledge, a novel carotenoid carrier protein, COCP, which consists of dimerized C-domain(s) of OCP and can combine with the isolated N-domain to form transient OCP-like species. Here, we demonstrate that in vitro COCP efficiently transfers otherwise tightly bound carotenoid to the full-length OCP apoprotein, resulting in formation of photoactive OCP from completely photoinactive species. We accurately analyze the peculiarities of this process that, to the best of our knowledge, appears unique, a previously uncharacterized protein-to-protein carotenoid transfer mechanism. We hypothesize that a similar OCP assembly can occur in vivo, substantiating specific roles of the COCP carotenoid carrier in cyanobacterial photoprotection.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Rakhimberdieva M.G., Stadnichuk I.N., Karapetyan N.V. Carotenoid-induced quenching of the phycobilisome fluorescence in photosystem II-deficient mutant of Synechocystis sp. FEBS Lett. 2004;574:85–88. - PubMed
-
- Kirilovsky D., Kerfeld C.A. The orange carotenoid protein in photoprotection of photosystem II in cyanobacteria. Biochim. Biophys. Acta. 2012;1817:158–166. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

