Adverse events in women and children who have received intrapartum antibiotic prophylaxis treatment: a systematic review
- PMID: 28747160
- PMCID: PMC5530570
- DOI: 10.1186/s12884-017-1432-3
Adverse events in women and children who have received intrapartum antibiotic prophylaxis treatment: a systematic review
Abstract
Background: Adverse events from intrapartum antibiotic prophylaxis (IAP) are poorly documented yet essential to inform clinical practice for neonatal group B Streptococcus (GBS) disease prevention. In this systematic review, we appraised and synthesised the evidence on the adverse events of IAP in the mother and/or her child.
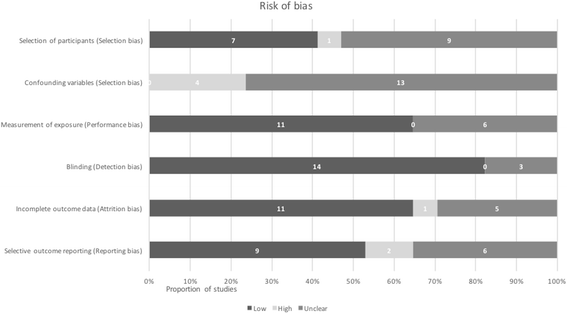
Methods: We searched MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Cochrane, and Science Citation Index from date of inception until October 16th 2016. Reference lists of included studies and relevant systematic reviews were hand-searched. We included primary studies in English that reported any adverse events from intrapartum antibiotics for any prophylactic purpose compared to controls. The search was not restricted to prophylaxis for GBS but excluded women with symptoms of infection or undergoing caesarean section. Two reviewers assessed the methodological quality of studies, using the Cochrane Risk of Bias tool, and the Risk of Bias Assessment Tool for Nonrandomised Studies. Results were synthesised narratively and displayed in text and tables.
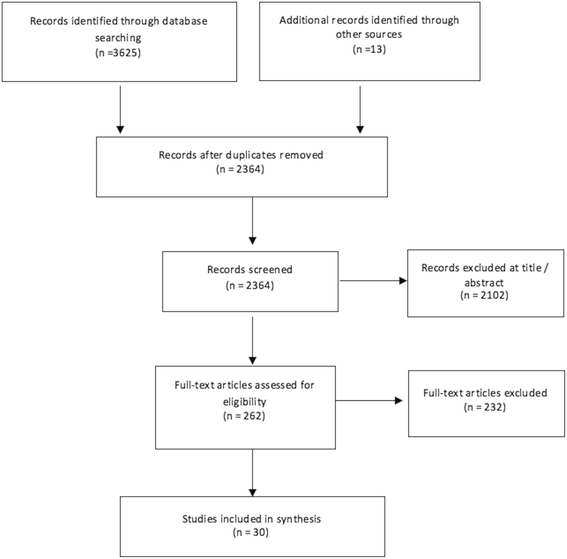
Results: From 2364 unique records, 30 studies were included. Despite a wide range of adverse events reported in 17 observational studies and 13 randomised controlled trials (RCTs), the evidence was inconsistent and at high risk of bias. Only one RCT investigated the long-term effects of IAP reporting potentially serious outcomes such as cerebral palsy; however, it had limited applicability and unclear biological plausibility. Seven observational studies showed that IAP for maternal GBS colonisation alters the infant microbiome. However, study populations were not followed through to clinical outcomes, therefore clinical significance is unknown. There was also observational evidence for increased antimicrobial resistance, however studies were at high or unclear risk of bias.
Conclusions: The evidence base to determine the frequency of adverse events from intrapartum antibiotic prophylaxis for neonatal GBS disease prevention is limited. As RCTs may not be possible, large, better quality, and longitudinal observational studies across countries with widespread IAP could fill this gap.
Trial registration: CRD42016037195 .
Keywords: Adverse events; Group B Streptococcus; Harms; Intrapartum antibiotic prophylaxis; Streptococcus agalactiae; Systematic review.
Conflict of interest statement
Authors’ information
FS is an experienced systematic reviewer, specialising in screening and infectious diseases. She is currently leading research using international data to understand the benefits and harms of GBS screening, leading the Cochrane systematic review on tests for maternal GBS carriage, has led a UK NSC review on international screening policy, and contributed to a health technology assessment for NICE. Dr. Julia Geppert, Dr. Chris Stinton, Dr. Jacoby Patterson, and Karoline Freeman are expert systematic reviewers specialising in screening and test accuracy, and have conducted health technology assessments for NICE and UK NSC policy reviews.
Dr. Tan is a consultant obstetrician & gynaecologist practising in the UK for 17 years, and has managed numerous patients with GBS in pregnancy. He is a Royal College of Obstetricians & Gynaecologists accredited subspecialist in Reproductive Medicine, a Member of the Royal College of Obstetricians & Gynaecologists (MRCOG) and Fellow of the Royal College of Surgeons (FRCS).
Professor Noel McCarthy is an academic public health physician and epidemiologist with qualifications including MPH, MSc (Medical Statistics) and DPhil (Epidemiology). He has over 60 peer-reviewed publications on public health issues in infectious disease control. Dr. Esther Robinson is the Lead Public Health Microbiologist for East Midlands Pubic Health England. She has a DPhil in Clinical Laboratory Sciences, and has experience with infection and antibiotic resistance. She is a fellow of the Royal College of Pathologists (FRCPath), member of the Royal College of Physicians (MRCP(UK)). Dr. Colin Brown is a Consultant in Infectious Diseases and Medical Microbiology at Public Health England with expertise in clinical infectious disease and disease policy.
Dr. Olalekan Uthman has worked across a wide range of health technology a focus on meta-analytical research and infectious diseases. He is an experienced Cochrane author and Cochrane Infectious Disease Group Editor and has contributed to several recent UK NICE public health and intervention guidelines and World Health Organization policies.
Samantha Johnson is an academic support librarian specialising in medicine and life sciences. Hannah Fraser has a Bachelor in Science, has studied the Masters in Screening course, and has provided administrative support to UK NSC policy reviews for health screening programmes.
Professor Aileen Clarke is a clinical public health academic who heads the Division of Health Sciences. She is a Fellow of the Higher Education Academy and a fellow of the Royal College of General Practitioners, has over 99 publications on evidence for clinical practice and policy and health technology assessment and has active links with national and international bodies such as the Nuffield Trust, the Faculty of Public Health Research Policy Committee, and the Society for Medical Decision Making. Professor Clarke leads one of nine Technology Assessment Review teams providing systematic reviews to NICE.
Dr. Sian Taylor-Phillips is an Associate Professor of Screening and Test Evaluation with wide experience in systematic reviews and cost effectiveness analysis specialising in population screening. She is well published in academic papers on population screening, and has led research in a range of screening programmes, including leading UK NSC policy reviews. She has also provided oral evidence to the Science and Technology Parliamentary Committee on Health Screening.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Edwards M, Baker C. Group B streptococcal infections. In:. In: Remington J, Klein J, editors. Infectious diseases of the fetus and newborn infant. Philadelphia: Saunders; 2001. p. 1091-1156.
-
- Regan JA, Klebanoff MA, Nugent RP. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal infections and prematurity study group. Obstet Gynecol. 1991;77(4):604–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous