Cells with Treg-specific FOXP3 demethylation but low CD25 are prevalent in autoimmunity
- PMID: 28747257
- PMCID: PMC5656572
- DOI: 10.1016/j.jaut.2017.07.009
Cells with Treg-specific FOXP3 demethylation but low CD25 are prevalent in autoimmunity
Abstract
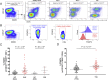
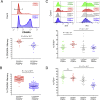
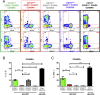
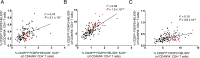
Identification of alterations in the cellular composition of the human immune system is key to understanding the autoimmune process. Recently, a subset of FOXP3+ cells with low CD25 expression was found to be increased in peripheral blood from systemic lupus erythematosus (SLE) patients, although its functional significance remains controversial. Here we find in comparisons with healthy donors that the frequency of FOXP3+ cells within CD127lowCD25low CD4+ T cells (here defined as CD25lowFOXP3+ T cells) is increased in patients affected by autoimmune disease of varying severity, from combined immunodeficiency with active autoimmunity, SLE to type 1 diabetes. We show that CD25lowFOXP3+ T cells share phenotypic features resembling conventional CD127lowCD25highFOXP3+ Tregs, including demethylation of the Treg-specific epigenetic control region in FOXP3, HELIOS expression, and lack of IL-2 production. As compared to conventional Tregs, more CD25lowFOXP3+HELIOS+ T cells are in cell cycle (33.0% vs 20.7% Ki-67+; P = 1.3 × 10-9) and express the late-stage inhibitory receptor PD-1 (67.2% vs 35.5%; P = 4.0 × 10-18), while having reduced expression of the early-stage inhibitory receptor CTLA-4, as well as other Treg markers, such as FOXP3 and CD15s. The number of CD25lowFOXP3+ T cells is correlated (P = 3.1 × 10-7) with the proportion of CD25highFOXP3+ T cells in cell cycle (Ki-67+). These findings suggest that CD25lowFOXP3+ T cells represent a subset of Tregs that are derived from CD25highFOXP3+ T cells, and are a peripheral marker of recent Treg expansion in response to an autoimmune reaction in tissues.
Keywords: Autoimmunity; CD25; FOXP3; Regulatory T cells (Tregs); Treg-specific demethylated region (TSDR).
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Ohkura N., Hamaguchi M., Morikawa H., Sugimura K., Tanaka A., Ito Y., Osaki M., Tanaka Y., Yamashita R., Nakano N., Huehn J., Fehling H.J., Sparwasser T., Nakai K., Sakaguchi S. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. - PubMed
-
- Miyara M., Yoshioka Y., Kitoh A., Shima T., Wing K., Niwa A., Parizot C., Taflin C., Heike T., Valeyre D., Mathian A., Nakahata T., Yamaguchi T., Nomura T., Ono M., Amoura Z., Gorochov G., Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. - PubMed
-
- Fuhrman C.A., Yeh W.-I., Seay H.R., Saikumar Lakshmi P., Chopra G., Zhang L., Perry D.J., McClymont S.A., Yadav M., Lopez M.-C., V Baker H., Zhang Y., Li Y., Whitley M., von Schack D., Atkinson M.A., Bluestone J.A., Brusko T.M. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J. Immunol. 2015;195:145–155. - PMC - PubMed
-
- Zhang B., Zhang X., Tang F.L., Zhu L.P., Liu Y., Lipsky P.E. Clinical significance of increased CD4+CD25−Foxp3+ T cells in patients with new-onset systemic lupus erythematosus. Ann. Rheum. Dis. 2008;67:1037–1040. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

