Embryo implantation evolved from an ancestral inflammatory attachment reaction
- PMID: 28747528
- PMCID: PMC5559003
- DOI: 10.1073/pnas.1701129114
Embryo implantation evolved from an ancestral inflammatory attachment reaction
Abstract
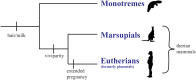
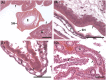
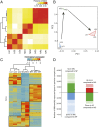
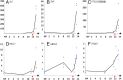
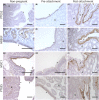
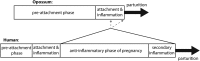
The molecular changes that support implantation in eutherian mammals are necessary to establish pregnancy. In marsupials, pregnancy is relatively short, and although a placenta does form, it is present for only a few days before parturition. However, morphological changes in the uterus of marsupials at term mimic those that occur during implantation in humans and mice. We investigated the molecular similarity between term pregnancy in the marsupials and implantation in eutherian mammals using the gray short-tailed opossum (Monodelphis domestica) as a model. Transcriptomic analysis shows that term pregnancy in the opossum is characterized by an inflammatory response consistent with implantation in humans and mice. This immune response is temporally correlated with the loss of the eggshell, and we used immunohistochemistry to report that this reaction occurs at the materno-fetal interface. We demonstrate that key markers of implantation, including Heparin binding EGF-like growth factor and Mucin 1, exhibit expression and localization profiles consistent with the pattern observed during implantation in eutherian mammals. Finally, we show that there are transcriptome-wide similarities between the opossum attachment reaction and implantation in rabbits and humans. Our data suggest that the implantation reaction that occurs in eutherians is derived from an attachment reaction in the ancestral therian mammal which, in the opossum, leads directly to parturition. Finally, we argue that the ability to shift from an inflammatory attachment reaction to a noninflammatory period of pregnancy was a key innovation in eutherian mammals that allowed an extended period of intimate placentation.
Keywords: evolution; inflammation; marsupial; placenta; pregnancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Reply to Liu: Inflammation before implantation both in evolution and development.Proc Natl Acad Sci U S A. 2018 Jan 2;115(1):E3-E4. doi: 10.1073/pnas.1717001115. Epub 2017 Dec 12. Proc Natl Acad Sci U S A. 2018. PMID: 29233940 Free PMC article. No abstract available.
-
Implantation in eutherians: Which came first, the inflammatory reaction or attachment?Proc Natl Acad Sci U S A. 2018 Jan 2;115(1):E1-E2. doi: 10.1073/iti0118115. Epub 2017 Dec 12. Proc Natl Acad Sci U S A. 2018. PMID: 29233941 Free PMC article. No abstract available.
References
-
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: Key pieces of the development puzzle. Science. 1994;266:1508–1518. - PubMed
-
- Murphy CR. Uterine receptivity and the plasma membrane transformation. Cell Res. 2004;14:259–267. - PubMed
-
- Sharkey AM, Smith SK. The endometrium as a cause of implantation failure. Best Pract Res Clin Obstet Gynaecol. 2003;17:289–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

