Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function
- PMID: 28748042
- PMCID: PMC5508361
- DOI: 10.1080/20002297.2017.1340085
Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function
Abstract
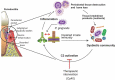
Porphyromonas gingivalis is a Gram-negative anaerobic rod that has been proposed as an orchestrator of complement-dependent dysbiotic inflammation. This notion was suggested from its capacities to manipulate the complement-Toll-like receptor crosstalk in ways that promote dysbiosis and periodontal disease in animal models. Specifically, while at low colonization levels, P. gingivalis interferes with innate immunity and leads to changes in the counts and composition of the oral commensal microbiota. The resulting dysbiotic microbial community causes disruption of host-microbial homeostasis, leading to inflammatory bone loss. These findings suggested that P. gingivalis can be considered as a keystone pathogen. The concept of keystone pathogens is one where their effects have community-wide significance and are disproportionate of their abundance. The present review summarizes the relevant literature and discusses whether the results from the animal models can be extrapolated to man.
Keywords: P. gingivalis; animal model; commensal microbiota; complement; keystone pathogen; periodontitis.
Figures

References
-
- Socransky SS, Haffajee AD.. Evidence of bacterial etiology: a historical perspective. Periodontol 2000. 1994;5:7–11. - PubMed
-
- Curtis M. The oral commensal microbiota bites back through Nod1. Cell Host Microbe. 2013;13:503–505. - PubMed
-
- Diaz PI, Hoare A, Hong BY. Subgingival microbiome shifts and community dynamics in periodontal diseases. J Calif Dent Assoc. 2016;44:421–435. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
