Lactadherin orthologs inhibit migration of human, porcine and murine intestinal epithelial cells
- PMID: 28748083
- PMCID: PMC5520951
- DOI: 10.1002/fsn3.479
Lactadherin orthologs inhibit migration of human, porcine and murine intestinal epithelial cells
Abstract
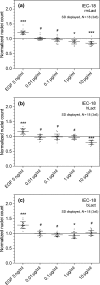
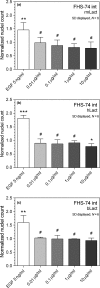
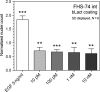
Lactadherin was originally described due to its appearance in milk, but is abundantly expressed especially by professional and nonprofessional phagocytes. The proteins has been shown to have a multitude of bioactive effects, including inhibition of inflammatory phospholipases, induction of effero- and phagocytosis, prevent rotavirus induced gastroenteritis, and modulate intestinal homeostasis by regulating epithelial cell migration. The level of expression seems to be important in a row of serious pathologies linked to the intestinal epithelial barrier function, vascular- and autoimmune disease. This study examines the ability of lactadherin to modulate migration of intestinal epithelium. A cell exclusion assay is used to quantify the ability of human, bovine and murine lactadherin orthologs to affect migration of primary small intestine epithelium cells. Previous reports show that recombinant murine lactadherin stimulate rat small intestine cell migration. The present study could not confirm this. Conversely, 10 μg/ml lactadherin inhibits migration. Therefore, as lactadherins enteroprotective properties is well established using in vivo models we conclude that the protective effects are linked to lactadherins ability operate as an opsonin, or other modulating effects, and not a direct lactadherin-cell induction of migration. Thus, the molecular mechanism behind the enteroprotective role of lactadherin remains to be established.
Keywords: IBD; MFG‐E8; intestinal cell migration; lactadherin; wound healing.
Figures




Similar articles
-
Effects of lactadherin on plasma D-lactic acid and small intestinal MUC2 and claudin-1 expression levels in rats with rotavirus-induced diarrhea.Exp Ther Med. 2016 Mar;11(3):943-950. doi: 10.3892/etm.2016.3015. Epub 2016 Jan 22. Exp Ther Med. 2016. PMID: 26998017 Free PMC article.
-
Role of lactadherin in intestinal barrier integrity in experimental neonatal necrotizing enterocolitis.J Cell Biochem. 2019 Dec;120(12):19509-19517. doi: 10.1002/jcb.29255. Epub 2019 Jul 2. J Cell Biochem. 2019. PMID: 31265168
-
Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion.DNA Cell Biol. 1997 Jul;16(7):861-9. doi: 10.1089/dna.1997.16.861. DNA Cell Biol. 1997. PMID: 9260929
-
Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns.Int J Biochem Cell Biol. 2013 Aug;45(8):1730-47. doi: 10.1016/j.biocel.2013.04.028. Epub 2013 May 6. Int J Biochem Cell Biol. 2013. PMID: 23660296 Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Identification of endogenous microRNA references in porcine serum for quantitative real-time PCR normalization.Mol Biol Rep. 2018 Oct;45(5):943-949. doi: 10.1007/s11033-018-4242-4. Epub 2018 Jul 25. Mol Biol Rep. 2018. PMID: 30047037
-
Human cell-expressed tag-free rhMFG-E8 as an effective radiation mitigator.Sci Rep. 2023 Dec 13;13(1):22186. doi: 10.1038/s41598-023-49499-y. Sci Rep. 2023. PMID: 38092894 Free PMC article.
References
-
- Andersen, M. H. , Berglund, L. , Rasmussen, J. T. , & Petersen, T. E. (1997). Bovine PAS‐6/7 binds alpha v beta 5 integrins and anionic phospholipids through two domains. Biochemistry, 36, 5441–5446. - PubMed
-
- Andersen, M. H. , Graversen, H. , Fedosov, S. N. , Petersen, T. E. , & Rasmussen, J. T . (2000). Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry, 39, 6200–6206. - PubMed
-
- Aziz, M. M. , Ishihara, S. , Mishima, Y. , Oshima, N. , Moriyama, I. , Yuki, T. , Kadowaki, Y. , Rumi, M. A. , Amano, Y. , & Kinoshita, Y. (2009). MFG‐E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin‐dependent alphavbeta3 integrin signaling. Journal of Immunology, 182, 7222–7232. - PubMed
-
- Aziz, M. , Jacob, A. , Matsuda, A. , & Wang, P. (2011). Review: milk fat globule‐EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis, 16, 1077–1086. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

