Novel Actions of Growth Hormone in Podocytes: Implications for Diabetic Nephropathy
- PMID: 28748185
- PMCID: PMC5506074
- DOI: 10.3389/fmed.2017.00102
Novel Actions of Growth Hormone in Podocytes: Implications for Diabetic Nephropathy
Abstract
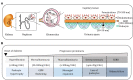
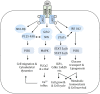
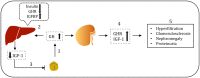
The kidney regulates water, electrolyte, and acid-base balance and thus maintains body homeostasis. The kidney's potential to ensure ultrafiltered and almost protein-free urine is compromised in various metabolic and hormonal disorders such as diabetes mellitus (DM). Diabetic nephropathy (DN) accounts for ~20-40% of mortality in DM. Proteinuria, a hallmark of renal glomerular diseases, indicates injury to the glomerular filtration barrier (GFB). The GFB is composed of glomerular endothelium, basement membrane, and podocytes. Podocytes are terminally differentiated epithelial cells with limited ability to replicate. Podocyte shape and number are both critical for the integrity and function of the GFB. Podocytes are vulnerable to various noxious stimuli prevalent in a diabetic milieu that could provoke podocytes to undergo changes to their unique architecture and function. Effacement of podocyte foot process is a typical morphological alteration associated with proteinuria. The dedifferentiation of podocytes from epithelial-to-mesenchymal phenotype and consequential loss results in proteinuria. Poorly controlled type 1 DM is associated with elevated levels of circulating growth hormone (GH), which is implicated in the pathophysiology of various diabetic complications including DN. Recent studies demonstrate that functional GH receptors are expressed in podocytes and that GH may exert detrimental effects on the podocyte. In this review, we summarize recent advances that shed light on actions of GH on the podocyte that could play a role in the pathogenesis of DN.
Keywords: dedifferentiation; diabetic nephropathy; growth hormone; hypertrophy; podocytes; zinc finger E-box binding homeobox2.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

