Epidemiology of heart failure in Germany: a retrospective database study
- PMID: 28748265
- PMCID: PMC5655572
- DOI: 10.1007/s00392-017-1137-7
Epidemiology of heart failure in Germany: a retrospective database study
Abstract
Background: Chronic heart failure (HF) is associated with significant healthcare expenditure, morbidity, and mortality. This study investigated the epidemiology of HF in Germany.
Methods: This retrospective study used anonymous healthcare claims data from the German Health Risk Institute on individuals with statutory health insurance. Patients with uninterrupted data from 1 January 2009 to 31 December 2013 or death (whichever occurred first), and ≥2 recorded HF-related diagnoses in 2011, were included. Patients with newly diagnosed HF were identified. Patients were followed up for 2 years from first diagnosis.
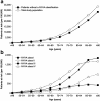
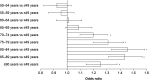
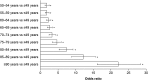
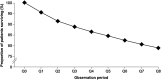
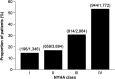
Results: Of 3,132,337 eligible patients, 123,925 (55.0% women; mean age 76.2 years) had HF: a prevalence of 3.96%. Of these, 26,368 had newly diagnosed HF: an incidence of 655/100,000 persons at risk. Incidence increased with age and was similar regardless of sex. During follow-up, there were 48,159 hospital admissions among newly diagnosed patients (1.8 hospitalizations/patient/2 years); HF accounted for 6% of these. Additionally, 20,148 patients (16.3%) overall and 5983 newly diagnosed patients (22.7%) died. Most new cases of HF were diagnosed by office-based physicians (63.2%); new cases among hospital inpatients were predominantly diagnosed by internal medicine specialists (70.7%). Overall, 94.0% received their initial prescription for HF treatment from a family practitioner.
Conclusions: The high prevalence and incidence observed in this representative sample emphasize the burden of HF in Germany. Substantial hospitalization rates and mortality highlight the need for early diagnosis and appropriate treatment, and for close cooperation between physician specialties and healthcare sectors.
Keywords: Epidemiology; Germany; Heart failure; Incidence; Prevalence.
Conflict of interest statement
Funding statement
This study was funded by Novartis Pharma AG. Data analysis was performed by Elsevier Health Analytics in coordination with the Health Risk Institute (both Berlin, Germany).
Ethical standards
This study was conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practices of the International Society for Pharmacoepidemiology (2008), the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (2008), and with the ethical principles laid down in the Declaration of Helsinki.
Conflict of interest
Stefan Störk receives funding from the German Federal Ministry of Education and Research (01EO1004 and 01EO1504) and has received honoraria from Bayer, Boehringer Ingelheim, Novartis, and Servier. Renate Handrock, Stephan Hupfer, and Sven Klebs are employees of Novartis Pharma GmbH. Josephine Jacob and Jochen Walker were employees of Elsevier Health Analytics at the time of the study and were consultants for Novartis. Frederico Calado and Raquel Lahoz are employees of Novartis Pharma AG.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

