Digital droplet PCR (ddPCR) for the detection and quantification of HPV 16, 18, 33 and 45 - a short report
- PMID: 28748500
- PMCID: PMC5608796
- DOI: 10.1007/s13402-017-0331-y
Digital droplet PCR (ddPCR) for the detection and quantification of HPV 16, 18, 33 and 45 - a short report
Abstract
Purpose: Human papilloma virus (HPV) infection is associated with several anogenital malignancies. Here, we set out to evaluate digital droplet PCR (ddPCR) as a tool for HPV 16, 18, 33 and 45 viral load quantification and, in addition, to compare the efficacy of the ddPCR assay for HPV 16 detection with that of quantitative real-time PCR (qPCR).
Methods: Clinical samples, positive for HPV genotypes 16, 18, 33 and 45 were analyzed for viral load using ddPCR. Sample DNA was cleaved before droplet generation and PCR. Droplets positive for VIC and FAM fluorescence were read in a QX200 Droplet reader™ (BIO-RAD) after which the viral load was calculated using Quantasoft software.
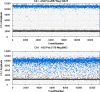
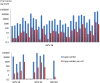
Results: We found that DNAs extracted from formalin fixed paraffin embedded (FFPE) tissue samples yielded lower amplification signals compared to those obtained from liquid based cytology (LBC) samples, but they were clearly distinguishable from negative background signals. The viral limit of detection was 1.6 copies of HPV 16, 2.8 copies of HPV 18, 4.6 copies of HPV 33 and 1.6 copies of HPV 45. The mean inter-assay coefficients of variability (CV) for the assays ranged from 3.4 to 7.0%, and the mean intra-assay CV from 2.6 to 8.2%. The viral load in the different cohorts of tumor samples ranged from 154 to 340,200 copies for HPV 16, 244 to 31,300 copies for HPV 18 and 738 to 69,100 copies for HPV 33. One sample positive for HPV 45 contained 1331 viral copies. When comparing qPCR data with ddPCR copy number data, the qPCR values were found to be 1 to 31 times higher.
Conclusions: Separation of fragments in nanodroplets may facilitate the amplification of fragmented human and viral DNA. The method of digital droplet PCR may, thus, provide a new and promising tool for evaluating the HPV viral load in clinical samples.
Keywords: Human papilloma virus (HPV); PCR; Quantitation; Viral load.
Conflict of interest statement
Conflict of interest
The authors have no conflicts of interests to disclose.
Funding
This study was funded by the Region Örebro County through ALF research funding and by the Örebro County Council Research Committee.
Ethical approval
All procedures were performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For the method optimization part, samples and results were coded and anonymized and could not be connected to either personal data or clinical evaluation. For the cohort study samples, approval was granted by the regional ethics committee board in Uppsala (Dnr 2008/294).
Informed consent
For the method optimization part, no informed consent was obtained since anonymized patient samples without any connection to patient data or patient identity were used. For the cohort study samples, informed consent was provided as specified in the ethical approval.
Figures

Similar articles
-
Droplet digital PCR (ddPCR) vs quantitative real-time PCR (qPCR) approach for detection and quantification of Merkel cell polyomavirus (MCPyV) DNA in formalin fixed paraffin embedded (FFPE) cutaneous biopsies.J Virol Methods. 2017 Aug;246:15-20. doi: 10.1016/j.jviromet.2017.04.003. Epub 2017 Apr 14. J Virol Methods. 2017. PMID: 28414163
-
Human Papillomavirus DNA Detection by Droplet Digital PCR in Formalin-Fixed Paraffin-Embedded Tumor Tissue from Oropharyngeal Squamous Cell Carcinoma Patients.Mol Diagn Ther. 2021 Jan;25(1):59-70. doi: 10.1007/s40291-020-00502-6. Epub 2020 Nov 27. Mol Diagn Ther. 2021. PMID: 33245553
-
Laboratory-developed L1 sequencing and type-specific, real-time polymerase chain reaction for the detection and typing of human papillomaviruses in formalin-fixed, paraffin-embedded tissues.Arch Pathol Lab Med. 2013 Jan;137(1):50-4. doi: 10.5858/arpa.2011-0392-OA. Arch Pathol Lab Med. 2013. PMID: 23276174
-
HPV detection methods and genotyping techniques in screening for cervical cancer.Ann Pathol. 2012 Dec;32(6):e15-23, 401-9. doi: 10.1016/j.annpat.2012.09.231. Epub 2012 Nov 22. Ann Pathol. 2012. PMID: 23244480 Review. English, French.
-
Droplet digital PCR of viral DNA/RNA, current progress, challenges, and future perspectives.J Med Virol. 2021 Jul;93(7):4182-4197. doi: 10.1002/jmv.26846. Epub 2021 Mar 11. J Med Virol. 2021. PMID: 33538349 Free PMC article. Review.
Cited by
-
Development of real-time PCR and droplet digital PCR based marker for the detection of Tilletia caries inciting common bunt of wheat.Front Plant Sci. 2022 Nov 25;13:1031611. doi: 10.3389/fpls.2022.1031611. eCollection 2022. Front Plant Sci. 2022. PMID: 36507438 Free PMC article.
-
Droplet digital PCR as alternative to microbiological culture for Mycobacterium tuberculosis complex detection in bovine lymph node tissue samples.Front Cell Infect Microbiol. 2024 Feb 26;14:1349999. doi: 10.3389/fcimb.2024.1349999. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38469351 Free PMC article.
-
Advances in human papillomavirus detection for cervical cancer screening and diagnosis: challenges of conventional methods and opportunities for emergent tools.Anal Methods. 2025 Feb 13;17(7):1428-1450. doi: 10.1039/d4ay01921k. Anal Methods. 2025. PMID: 39775553 Free PMC article. Review.
-
False Negative Results in Cervical Cancer Screening-Risks, Reasons and Implications for Clinical Practice and Public Health.Diagnostics (Basel). 2022 Jun 20;12(6):1508. doi: 10.3390/diagnostics12061508. Diagnostics (Basel). 2022. PMID: 35741319 Free PMC article. Review.
-
Frequent traces of EBV infection in Hodgkin and non-Hodgkin lymphomas classified as EBV-negative by routine methods: expanding the landscape of EBV-related lymphomas.Mod Pathol. 2020 Dec;33(12):2407-2421. doi: 10.1038/s41379-020-0575-3. Epub 2020 Jun 1. Mod Pathol. 2020. PMID: 32483241 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

