Digital droplet PCR (ddPCR) for the detection and quantification of HPV 16, 18, 33 and 45 - a short report
- PMID: 28748500
- PMCID: PMC5608796
- DOI: 10.1007/s13402-017-0331-y
Digital droplet PCR (ddPCR) for the detection and quantification of HPV 16, 18, 33 and 45 - a short report
Abstract
Purpose: Human papilloma virus (HPV) infection is associated with several anogenital malignancies. Here, we set out to evaluate digital droplet PCR (ddPCR) as a tool for HPV 16, 18, 33 and 45 viral load quantification and, in addition, to compare the efficacy of the ddPCR assay for HPV 16 detection with that of quantitative real-time PCR (qPCR).
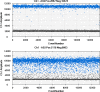
Methods: Clinical samples, positive for HPV genotypes 16, 18, 33 and 45 were analyzed for viral load using ddPCR. Sample DNA was cleaved before droplet generation and PCR. Droplets positive for VIC and FAM fluorescence were read in a QX200 Droplet reader™ (BIO-RAD) after which the viral load was calculated using Quantasoft software.
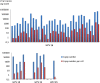
Results: We found that DNAs extracted from formalin fixed paraffin embedded (FFPE) tissue samples yielded lower amplification signals compared to those obtained from liquid based cytology (LBC) samples, but they were clearly distinguishable from negative background signals. The viral limit of detection was 1.6 copies of HPV 16, 2.8 copies of HPV 18, 4.6 copies of HPV 33 and 1.6 copies of HPV 45. The mean inter-assay coefficients of variability (CV) for the assays ranged from 3.4 to 7.0%, and the mean intra-assay CV from 2.6 to 8.2%. The viral load in the different cohorts of tumor samples ranged from 154 to 340,200 copies for HPV 16, 244 to 31,300 copies for HPV 18 and 738 to 69,100 copies for HPV 33. One sample positive for HPV 45 contained 1331 viral copies. When comparing qPCR data with ddPCR copy number data, the qPCR values were found to be 1 to 31 times higher.
Conclusions: Separation of fragments in nanodroplets may facilitate the amplification of fragmented human and viral DNA. The method of digital droplet PCR may, thus, provide a new and promising tool for evaluating the HPV viral load in clinical samples.
Keywords: Human papilloma virus (HPV); PCR; Quantitation; Viral load.
Conflict of interest statement
Conflict of interest
The authors have no conflicts of interests to disclose.
Funding
This study was funded by the Region Örebro County through ALF research funding and by the Örebro County Council Research Committee.
Ethical approval
All procedures were performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For the method optimization part, samples and results were coded and anonymized and could not be connected to either personal data or clinical evaluation. For the cohort study samples, approval was granted by the regional ethics committee board in Uppsala (Dnr 2008/294).
Informed consent
For the method optimization part, no informed consent was obtained since anonymized patient samples without any connection to patient data or patient identity were used. For the cohort study samples, informed consent was provided as specified in the ethical approval.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

