Concise Review: Stem Cells for Corneal Wound Healing
- PMID: 28748596
- PMCID: PMC5637932
- DOI: 10.1002/stem.2667
Concise Review: Stem Cells for Corneal Wound Healing
Abstract
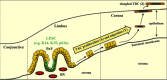
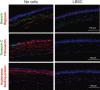
Corneal wound healing is a complex process that occurs in response to various injuries and commonly used refractive surgery. It is a significant clinical problem, which may lead to serious complications due to either incomplete (epithelial) or excessive (stromal) healing. Epithelial stem cells clearly play a role in this process, whereas the contribution of stromal and endothelial progenitors is less well studied. The available evidence on stem cell participation in corneal wound healing is reviewed, together with the data on the use of corneal and non-corneal stem cells to facilitate this process in diseased or postsurgical conditions. Important aspects of corneal stem cell generation from alternative cell sources, including pluripotent stem cells, for possible transplantation upon corneal injuries or in disease conditions are also presented. Stem Cells 2017;35:2105-2114.
Keywords: Cell transplantation; Corneal endothelium; Corneal epithelium; Gene therapy; Keratocyte; Pluripotent stem cell; Stem cell; Wound healing.
© 2017 The Authors STEM CELLS published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures



References
-
- Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 1971;229:560–561. - PubMed
-
- Tseng SC. Regulation and clinical implications of corneal epithelial stem cells. Mol Biol Rep 1996;23:47–58. - PubMed
-
- Shortt AJ, Secker GA, Munro PM et al. Characterization of the limbal epithelial stem cell niche: Novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells 2007;25:1402–1409. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

