Oesophageal cancer
- PMID: 28748917
- PMCID: PMC6168059
- DOI: 10.1038/nrdp.2017.48
Oesophageal cancer
Abstract
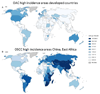
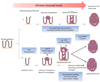
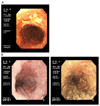
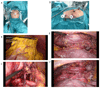
Oesophageal cancer is the sixth most common cause of cancer-related death worldwide and is therefore a major global health challenge. The two major subtypes of oesophageal cancer are oesophageal squamous cell carcinoma (OSCC) and oesophageal adenocarcinoma (OAC), which are epidemiologically and biologically distinct. OSCC accounts for 90% of all cases of oesophageal cancer globally and is highly prevalent in the East, East Africa and South America. OAC is more common in developed countries than in developing countries. Preneoplastic lesions are identifiable for both OSCC and OAC; these are frequently amenable to endoscopic ablative therapies. Most patients with oesophageal cancer require extensive treatment, including chemotherapy, chemoradiotherapy and/or surgical resection. Patients with advanced or metastatic oesophageal cancer are treated with palliative chemotherapy; those who are human epidermal growth factor receptor 2 (HER2)-positive may also benefit from trastuzumab treatment. Immuno-oncology therapies have also shown promising early results in OSCC and OAC. In this Primer, we review state-of-the-art knowledge on the biology and treatment of oesophageal cancer, including screening, endoscopic ablative therapies and emerging molecular targets, and we discuss best practices in chemotherapy, chemoradiotherapy, surgery and the maintenance of patient quality of life.
Conflict of interest statement
ES declares honararia for advisory role from Five Prime Therapeutics and Bristol-Meyer Squibb. DC declares institutional research funding from Amgen, AstraZeneca, Bayer, Celgene, MedImmune, Merck Serono, Merrimack, and Sanofi. FL has received research support from GlaxoSmithKline and Fresenius Biotech; lecture and advisory honoraria from Amgen, Biontech, Bristol-Myers Squibb, Eli Lilly, Ganymed, Merck-Serono, MSD, Nordic and Roche; travel support from Amgen, Bayer, Roche and Taiho. MAS declares honararia for an adivory role from Lilly and institutional research Funding from Genentech and Sanofi. JL, PL, RF declare no conflicts.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

