Hair follicle-associated-pluripotent (HAP) stem cells
- PMID: 28749199
- PMCID: PMC5736337
- DOI: 10.1080/15384101.2017.1356513
Hair follicle-associated-pluripotent (HAP) stem cells
Abstract
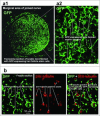
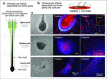
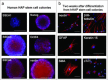
Various types of stem cells reside in the skin, including keratinocyte progenitor cells, melanocyte progenitor cells, skin-derived precursors (SKPs), and nestin-expressing hair follicle-associated-pluripotent (HAP) stem cells. HAP stem cells, located in the bulge area of the hair follicle, have been shown to differentiate to nerve cells, glial cells, keratinocytes, smooth muscle cells, cardiac muscle cells, and melanocytes. HAP stem cells are positive for the stem-cell marker CD34, as well as K15-negative, suggesting their relatively undifferentiated state. Therefore, HAP stem cells may be the most primitive stem cells in the skin. Moreover, HAP stem cells can regenerate the epidermis and at least parts of the hair follicle. These results suggest that HAP stem cells may be the origin of other stem cells in the skin. Transplanted HAP stem cells promote the recovery of peripheral-nerve and spinal-cord injuries and have the potential for heart regeneration as well. HAP stem cells are readily accessible from everyone, do not form tumors, and can be cryopreserved without loss of differentiation potential. These results suggest that HAP stem cells may have greater potential than iPS or ES cells for regenerative medicine.
Keywords: Hair follicle; bulge area; cardiac muscle cell; differentiation; nestin; neuron; stem cell.
Figures


References
-
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–48. https://doi.org/10.1016/j.cell.2004.08.012. PMID:15339667. - DOI - PubMed
-
- Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, Herlyn M, Xu X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168:1879–88. https://doi.org/10.2353/ajpath.2006.051170. PMID:16723703. - DOI - PMC - PubMed
-
- Mignone JL, Roig-Lopez JL, Fedtsova N, Schones DE, Manganas LN, Maletic-Savatic M, Keyes WM, Mills AA, Gleiberman A, Zhang MQ, Enikolopov G. Neural potential of a stem cell population in the hair follicle. Cell Cycle. 2007;6:2161–70. https://doi.org/10.4161/cc.6.17.4593. PMID:17873521. - DOI - PMC - PubMed
-
- Cotsarelis G, Sun T, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–37. https://doi.org/10.1016/0092-8674(90)90696-C. PMID:2364430. - DOI - PubMed
-
- Nishimura EK, Jordan JA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–60. https://doi.org/10.1038/416854a. PMID:11976685. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
