Keratin 23 promotes telomerase reverse transcriptase expression and human colorectal cancer growth
- PMID: 28749462
- PMCID: PMC5550880
- DOI: 10.1038/cddis.2017.339
Keratin 23 promotes telomerase reverse transcriptase expression and human colorectal cancer growth
Abstract
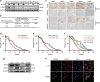
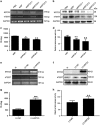
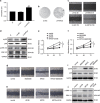
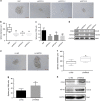
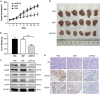
The overexpression of human telomerase reverse transcriptase (hTERT) has been associated with the proliferation and migration of colorectal cancer (CRC) cells. We investigated the roles of KRT23 and hTERT in promoting CRC cell proliferation and migration. We verified the relationship between KRT23 and hTERT in CRC using streptavidin-agarose pulldown and chromatin immunoprecipitation (ChIP) assays. One hundred and fifty-four human CRC specimens were analyzed using immunohistochemistry. The roles of KRT23 and hTERT in cell growth and migration were studied using siRNA and lentiviruses in vivo and in vitro. Western blot and wound scratch analyses were used to determine the signaling pathway for KRT23-mediated activation of CRC growth and migration. Telomerase activity was measured by using the TeloTAGGG Telomerase PCR ELISA PLUS Kit. We identified KRT23 as a new hTERT promoter-binding protein. Patients with high KRT23 and hTERT expression had markedly shorter overall survival. Overexpression of KRT23 upregulated the expression of hTERT protein, hTERT promoter-driven luciferase and telomerase activity in CRC. Conversely, inhibition of KRT23 by a KRT23-specific siRNA repressed the endogenous hTERT protein, the expression of hTERT promoter-driven luciferase and telomerase activity. Overexpression of KRT23 also promoted CRC proliferation and migration. By contrast, KRT23 inhibition significantly inhibited tumor cell growth in vitro and in vivo. KRT23 promoted cancer stem cell properties and increased the expression of CD133 and CD44. These results demonstrate that KRT23 is an important cellular factor that promotes CRC growth by activating hTERT expression and that KRT23 is a potential novel therapeutic target for CRC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
CDC5L Promotes hTERT Expression and Colorectal Tumor Growth.Cell Physiol Biochem. 2017;41(6):2475-2488. doi: 10.1159/000475916. Epub 2017 May 4. Cell Physiol Biochem. 2017. PMID: 28472785
-
Human telomerase reverse transcriptase recruits the β-catenin/TCF-4 complex to transactivate chemokine (C-C motif) ligand 2 expression in colorectal cancer.Biomed Pharmacother. 2019 Apr;112:108700. doi: 10.1016/j.biopha.2019.108700. Epub 2019 Feb 26. Biomed Pharmacother. 2019. PMID: 30970512
-
Ret finger protein-like 3 promotes tumor cell growth by activating telomerase reverse transcriptase expression in human lung cancer cells.Oncotarget. 2014 Dec 15;5(23):11909-23. doi: 10.18632/oncotarget.2557. Oncotarget. 2014. PMID: 25481043 Free PMC article.
-
Essential roles of telomerase reverse transcriptase hTERT in cancer stemness and metastasis.FEBS Lett. 2018 Jun;592(12):2023-2031. doi: 10.1002/1873-3468.13084. Epub 2018 May 18. FEBS Lett. 2018. PMID: 29749098 Review.
-
Study of the role of telomerase in colorectal cancer: preliminary report and literature review.G Chir. 2017 Sep-Oct;38(5):213-218. doi: 10.11138/gchir/2017.38.5.213. G Chir. 2017. PMID: 29280699 Free PMC article. Review.
Cited by
-
A novel 12-gene signature as independent prognostic model in stage IA and IB lung squamous cell carcinoma patients.Clin Transl Oncol. 2021 Nov;23(11):2368-2381. doi: 10.1007/s12094-021-02638-1. Epub 2021 May 24. Clin Transl Oncol. 2021. PMID: 34028782
-
An in vitro vascularized micro-tumor model of human colorectal cancer recapitulates in vivo responses to standard-of-care therapy.Lab Chip. 2021 Apr 7;21(7):1333-1351. doi: 10.1039/d0lc01216e. Epub 2021 Feb 19. Lab Chip. 2021. PMID: 33605955 Free PMC article.
-
Does side matter? Deciphering mechanisms that underpin side-dependent pathogenesis and therapy response in colorectal cancer.Mol Cancer. 2025 May 2;24(1):130. doi: 10.1186/s12943-025-02327-5. Mol Cancer. 2025. PMID: 40312719 Free PMC article. Review.
-
PPP1R14B is a diagnostic prognostic marker in patients with uterine corpus endometrial carcinoma.J Cell Mol Med. 2023 Mar;27(6):846-863. doi: 10.1111/jcmm.17697. Epub 2023 Feb 23. J Cell Mol Med. 2023. PMID: 36824011 Free PMC article.
-
Study of anticancer effects of platinum levetiracetam and levetiracetam via cancer biomarkers genes expression on HepG2 cell line.Mol Biol Rep. 2023 Nov;50(11):9431-9439. doi: 10.1007/s11033-023-08890-8. Epub 2023 Oct 13. Mol Biol Rep. 2023. PMID: 37831345
References
-
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014; 383: 1490–1502. - PubMed
-
- Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O'Connor E et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315: 2576–2594. - PubMed
-
- Blasco MA. Telomeres and cancer: a tale with many endings. Curr Opin Genet Dev 2003; 13: 70–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

