The wide genetic landscape of clinical frontotemporal dementia: systematic combined sequencing of 121 consecutive subjects
- PMID: 28749476
- PMCID: PMC5846812
- DOI: 10.1038/gim.2017.102
The wide genetic landscape of clinical frontotemporal dementia: systematic combined sequencing of 121 consecutive subjects
Abstract
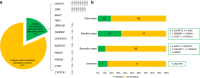
PurposeTo define the genetic spectrum and relative gene frequencies underlying clinical frontotemporal dementia (FTD).MethodsWe investigated the frequencies and mutations in neurodegenerative disease genes in 121 consecutive FTD subjects using an unbiased, combined sequencing approach, complemented by cerebrospinal fluid Aβ1-42 and serum progranulin measurements. Subjects were screened for C9orf72 repeat expansions, GRN and MAPT mutations, and, if negative, mutations in other neurodegenerative disease genes, by whole-exome sequencing (WES) (n = 108), including WES-based copy-number variant (CNV) analysis.ResultsPathogenic and likely pathogenic mutations were identified in 19% of the subjects, including mutations in C9orf72 (n = 8), GRN (n = 7, one 11-exon macro-deletion) and, more rarely, CHCHD10, TARDBP, SQSTM1 and UBQLN2 (each n = 1), but not in MAPT or TBK1. WES also unraveled pathogenic mutations in genes not commonly linked to FTD, including mutations in Alzheimer (PSEN1, PSEN2), lysosomal (CTSF, 7-exon macro-deletion) and cholesterol homeostasis pathways (CYP27A1).ConclusionOur unbiased approach reveals a wide genetic spectrum underlying clinical FTD, including 11% of seemingly sporadic FTD. It unravels several mutations and CNVs in genes and pathways hitherto not linked to FTD. This suggests that clinical FTD might be the converging downstream result of a delicate susceptibility of frontotemporal brain networks to insults in various pathways.
Conflict of interest statement
M.S. has received honoraria from Actelion Pharmaceuticals, unrelated to this work. C.H. is an adviser to F. Hoffmann–La Roche. The authors declare no conflict of interest related to the present work.
Figures


References
-
- Neary D, Snowden JS, Gustafson L et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

