Update: Interim Guidance for Health Care Providers Caring for Pregnant Women with Possible Zika Virus Exposure - United States (Including U.S. Territories), July 2017
- PMID: 28749921
- PMCID: PMC5657812
- DOI: 10.15585/mmwr.mm6629e1
Update: Interim Guidance for Health Care Providers Caring for Pregnant Women with Possible Zika Virus Exposure - United States (Including U.S. Territories), July 2017
Abstract
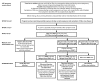
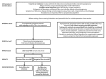
CDC has updated the interim guidance for U.S. health care providers caring for pregnant women with possible Zika virus exposure in response to 1) declining prevalence of Zika virus disease in the World Health Organization's Region of the Americas (Americas) and 2) emerging evidence indicating prolonged detection of Zika virus immunoglobulin M (IgM) antibodies. Zika virus cases were first reported in the Americas during 2015-2016; however, the incidence of Zika virus disease has since declined. As the prevalence of Zika virus disease declines, the likelihood of false-positive test results increases. In addition, emerging epidemiologic and laboratory data indicate that, as is the case with other flaviviruses, Zika virus IgM antibodies can persist beyond 12 weeks after infection. Therefore, IgM test results cannot always reliably distinguish between an infection that occurred during the current pregnancy and one that occurred before the current pregnancy, particularly for women with possible Zika virus exposure before the current pregnancy. These limitations should be considered when counseling pregnant women about the risks and benefits of testing for Zika virus infection during pregnancy. This updated guidance emphasizes a shared decision-making model for testing and screening pregnant women, one in which patients and providers work together to make decisions about testing and care plans based on patient preferences and values, clinical judgment, and a balanced assessment of risks and expected outcomes.
Conflict of interest statement
Figures
References
-
- Pan American Health Organization. Regional Zika epidemiological update (Americas) May 25, 2017. Washington, DC: Pan American Health Organization. http://www.paho.org/hq/index.php?option=com_content&view=article&id=1159...
-
- World Health Organization. Zika virus country classification scheme: interim guidance. Geneva, Switzerland: World Health Organization, 2017. http://www.who.int/csr/resources/publications/zika/classification/en/
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

