Chemoenzymatic synthesis of glycoengineered IgG antibodies and glycosite-specific antibody-drug conjugates
- PMID: 28749929
- PMCID: PMC5705183
- DOI: 10.1038/nprot.2017.058
Chemoenzymatic synthesis of glycoengineered IgG antibodies and glycosite-specific antibody-drug conjugates
Abstract
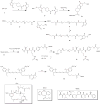
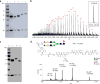
Glycoengineered therapeutic antibodies and glycosite-specific antibody-drug conjugates (gsADCs) have generated great interest among researchers because of their therapeutic potential. Endoglycosidase-catalyzed in vitro glycoengineering technology is a powerful tool for IgG Fc (fragment cystallizable) N-glycosylation remodeling. In this protocol, native heterogeneously glycosylated IgG N-glycans are first deglycosylated with a wild-type endoglycosidase. Next, a homogeneous N-glycan substrate, presynthesized as described here, is attached to the remaining N-acetylglucosamine (GlcNAc) of IgG, using a mutant endoglycosidase (also called endoglycosynthase) that lacks hydrolytic activity but possesses transglycosylation activity for glycoengineering. Compared with in vivo glycoengineering technologies and the glycosyltransferase-enabled in vitro engineering method, the current approach is robust and features quantitative yield, homogeneous glycoforms of produced antibodies and ADCs, compatibility with diverse natural and non-natural glycan structures, convenient exploitation of native IgG as the starting material, and a well-defined conjugation site for antibody modifications. Potential applications of this method cover a broad scope of antibody-related research, including the development of novel glycoengineered therapeutic antibodies with enhanced efficacy, site-specific antibody-drug conjugation, and site-specific modification of antibodies for fluorescent labeling, PEGylation, protein cross-linking, immunoliposome formation, and so on, without loss of antigen-binding affinity. It takes 5-8 d to prepare the natural or modified N-glycan substrates, 3-4 d to engineer the IgG N-glycosylation, and 2-5 d to synthesize the small-molecule toxins and prepare the gsADCs.
Conflict of interest statement
Figures




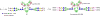

Similar articles
-
One-pot N-glycosylation remodeling of IgG with non-natural sialylglycopeptides enables glycosite-specific and dual-payload antibody-drug conjugates.Org Biomol Chem. 2016 Oct 12;14(40):9501-9518. doi: 10.1039/c6ob01751g. Org Biomol Chem. 2016. PMID: 27714198
-
Homogeneous Antibody-Drug Conjugates via Glycoengineering.Methods Mol Biol. 2019;2033:221-238. doi: 10.1007/978-1-4939-9654-4_15. Methods Mol Biol. 2019. PMID: 31332757
-
Chemoenzymatic Glyco-engineering of Monoclonal Antibodies.Methods Mol Biol. 2015;1321:375-87. doi: 10.1007/978-1-4939-2760-9_25. Methods Mol Biol. 2015. PMID: 26082235 Free PMC article.
-
Glycosylation engineering of therapeutic IgG antibodies: challenges for the safety, functionality and efficacy.Protein Cell. 2018 Jan;9(1):47-62. doi: 10.1007/s13238-017-0433-3. Epub 2017 Jun 8. Protein Cell. 2018. PMID: 28597152 Free PMC article. Review.
-
Crystallizable Fragment Glycoengineering for Therapeutic Antibodies Development.Front Immunol. 2017 Nov 13;8:1554. doi: 10.3389/fimmu.2017.01554. eCollection 2017. Front Immunol. 2017. PMID: 29181010 Free PMC article. Review.
Cited by
-
Oligosaccharide Synthesis and Translational Innovation.J Am Chem Soc. 2019 Mar 6;141(9):3735-3754. doi: 10.1021/jacs.8b11005. Epub 2019 Feb 18. J Am Chem Soc. 2019. PMID: 30716271 Free PMC article. Review.
-
A "One-Step" Strategy for the Global Characterization of Core-Fucosylated Glycoproteome.JACS Au. 2024 May 1;4(5):2005-2018. doi: 10.1021/jacsau.4c00214. eCollection 2024 May 27. JACS Au. 2024. PMID: 38818065 Free PMC article.
-
Glycoengineering of Antibodies for Modulating Functions.Annu Rev Biochem. 2019 Jun 20;88:433-459. doi: 10.1146/annurev-biochem-062917-012911. Epub 2019 Mar 27. Annu Rev Biochem. 2019. PMID: 30917003 Free PMC article. Review.
-
General and Robust Chemoenzymatic Method for Glycan-Mediated Site-Specific Labeling and Conjugation of Antibodies: Facile Synthesis of Homogeneous Antibody-Drug Conjugates.ACS Chem Biol. 2021 Nov 19;16(11):2502-2514. doi: 10.1021/acschembio.1c00597. Epub 2021 Sep 27. ACS Chem Biol. 2021. PMID: 34569782 Free PMC article.
-
Introduction of Carbonyl Groups into Antibodies.Molecules. 2023 Dec 1;28(23):7890. doi: 10.3390/molecules28237890. Molecules. 2023. PMID: 38067618 Free PMC article. Review.
References
-
- Shields RL, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. - PubMed
-
- Yamane-Ohnuki N, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614–622. - PubMed
-
- Shinkawa T, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. - PubMed
-
- Gramer MJ, et al. Modulation of antibody galactosylation through feeding of uridine, manganese chloride, and galactose. Biotechnol Bioeng. 2011;108:1591–1602. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

