Quantitative, multiplexed workflow for deep analysis of human blood plasma and biomarker discovery by mass spectrometry
- PMID: 28749931
- PMCID: PMC6057147
- DOI: 10.1038/nprot.2017.054
Quantitative, multiplexed workflow for deep analysis of human blood plasma and biomarker discovery by mass spectrometry
Abstract
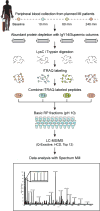
Proteomic characterization of blood plasma is of central importance to clinical proteomics and particularly to biomarker discovery studies. The vast dynamic range and high complexity of the plasma proteome have, however, proven to be serious challenges and have often led to unacceptable tradeoffs between depth of coverage and sample throughput. We present an optimized sample-processing pipeline for analysis of the human plasma proteome that provides greatly increased depth of detection, improved quantitative precision and much higher sample analysis throughput as compared with prior methods. The process includes abundant protein depletion, isobaric labeling at the peptide level for multiplexed relative quantification and ultra-high-performance liquid chromatography coupled to accurate-mass, high-resolution tandem mass spectrometry analysis of peptides fractionated off-line by basic pH reversed-phase (bRP) chromatography. The overall reproducibility of the process, including immunoaffinity depletion, is high, with a process replicate coefficient of variation (CV) of <12%. Using isobaric tags for relative and absolute quantitation (iTRAQ) 4-plex, >4,500 proteins are detected and quantified per patient sample on average, with two or more peptides per protein and starting from as little as 200 μl of plasma. The approach can be multiplexed up to 10-plex using tandem mass tags (TMT) reagents, further increasing throughput, albeit with some decrease in the number of proteins quantified. In addition, we provide a rapid protocol for analysis of nonfractionated depleted plasma samples analyzed in 10-plex. This provides ∼600 quantified proteins for each of the ten samples in ∼5 h of instrument time.
Figures


References
-
- Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. - PubMed
-
- Hortin GL, Jortani SA, Ritchie JC, Jr, Valdes R, Jr, Chan DW. Proteomics: a new diagnostic frontier. Clin Chem. 2006;52:1218–1222. - PubMed
-
- Pernemalm M, Lehtio J. Mass spectrometry-based plasma proteomics: state of the art and future outlook. Expert Rev Proteomics. 2014;11:431–448. - PubMed
-
- States DJ, et al. Challenges in deriving high-confidence protein identifications from data gathered by a HUPO plasma proteome collaborative study. Nat Biotechnol. 2006;24:333–338. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

