Upregulation of an inward rectifying K+ channel can rescue slow Ca2+ oscillations in K(ATP) channel deficient pancreatic islets
- PMID: 28749940
- PMCID: PMC5549769
- DOI: 10.1371/journal.pcbi.1005686
Upregulation of an inward rectifying K+ channel can rescue slow Ca2+ oscillations in K(ATP) channel deficient pancreatic islets
Abstract
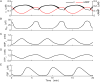
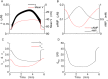
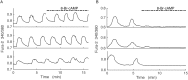
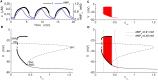
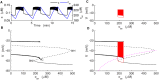
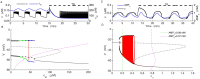
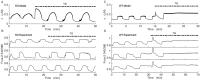
Plasma insulin oscillations are known to have physiological importance in the regulation of blood glucose. In insulin-secreting β-cells of pancreatic islets, K(ATP) channels play a key role in regulating glucose-dependent insulin secretion. In addition, they convey oscillations in cellular metabolism to the membrane by sensing adenine nucleotides, and are thus instrumental in mediating pulsatile insulin secretion. Blocking K(ATP) channels pharmacologically depolarizes the β-cell plasma membrane and terminates islet oscillations. Surprisingly, when K(ATP) channels are genetically knocked out, oscillations in islet activity persist, and relatively normal blood glucose levels are maintained. Compensation must therefore occur to overcome the loss of K(ATP) channels in K(ATP) knockout mice. In a companion study, we demonstrated a substantial increase in Kir2.1 protein occurs in β-cells lacking K(ATP) because of SUR1 deletion. In this report, we demonstrate that β-cells of SUR1 null islets have an upregulated inward rectifying K+ current that helps to compensate for the loss of K(ATP) channels. This current is likely due to the increased expression of Kir2.1 channels. We used mathematical modeling to determine whether an ionic current having the biophysical characteristics of Kir2.1 is capable of rescuing oscillations that are similar in period to those of wild-type islets. By experimentally testing a key model prediction we suggest that Kir2.1 current upregulation is a likely mechanism for rescuing the oscillations seen in islets from mice deficient in K(ATP) channels.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Pørksen N. The in vivo regulation of pulsatile insulin secretion. Diabetologia. 2002;45: 3–20. doi: 10.1007/s001250200001 - DOI - PubMed
-
- Nunemaker CS, Zhang M, Wasserman DH, McGuinness OP, Powers AC, Bertram R, et al. Individual mice can be distinguished by the period of their islet calcium oscillations: Is there an intrinsic islet period that is imprinted in vivo? Diabetes. 2005;54: 3517–3522. doi: 10.2337/diabetes.54.12.3517 - DOI - PubMed
-
- Song SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, Butler PC. Direct measurement of pulsatile insulin secretion form the portal vein in human subjects. J Clin Endocrinol Metab. 2007;85: 4491–4499. - PubMed
-
- Matveyenko AV, Veldhuis JD, Butler PC. Measurement of pulsatile insulin secretion in the rat: Direct sampling from the hepatic portal vein. Am J Physiol. 2008;295: E569–E574. doi: 10.1152/ajpendo.90335.2008 - DOI - PMC - PubMed
-
- Matthews DR, Naylor BA, Jones RG. Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes. 1983. 32(7): 617–621. doi: 10.2337/diabetes.32.7.617 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

