Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model
- PMID: 28750018
- PMCID: PMC5531481
- DOI: 10.1371/journal.pone.0182039
Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model
Abstract
Pancreatic cancer (PC) remains one of the most challenging solid tumors to treat with a high unmet medical need as patients poorly respond to standard-of-care-therapies. Prominent desmoplastic reaction involving cancer-associated fibroblasts (CAFs) and the immune cells in the tumor microenvironment (TME) and their cross-talk play a significant role in tumor immune escape and progression. To identify the key cellular mechanisms induce an immunosuppressive tumor microenvironment, we established 3D co-culture model with pancreatic cancer cells, CAFs and monocytes. Using this model, we analyzed the influence of tumor cells and fibroblasts on monocytes and their immune suppressive phenotype. Phenotypic characterization of the monocytes after 3D co-culture with tumor/fibroblast spheroids was performed by analyzing the expression of defined cell surface markers and soluble factors. Functionality of these monocytes and their ability to influence T cell phenotype and proliferation was investigated. 3D co-culture of monocytes with pancreatic cancer cells and fibroblasts induced the production of immunosuppressive cytokines which are known to promote polarization of M2 like macrophages and myeloid derived suppressive cells (MDSCs). These co-culture spheroid polarized monocyte derived macrophages (MDMs) were poorly differentiated and had an M2 phenotype. The immunosuppressive function of these co-culture spheroids polarized MDMs was demonstrated by their ability to inhibit CD4+ and CD8+ T cell activation and proliferation in vitro, which we could partially reverse by 3D co-culture spheroid treatment with therapeutic molecules that are able to re-activated spheroid polarized MDMs or block immune suppressive factors such as Arginase-I.
Conflict of interest statement
Figures


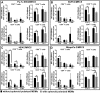
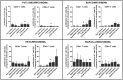
Similar articles
-
Cancer-associated fibroblasts promote M2 polarization of macrophages in pancreatic ductal adenocarcinoma.Cancer Med. 2017 Feb;6(2):463-470. doi: 10.1002/cam4.993. Epub 2017 Jan 18. Cancer Med. 2017. PMID: 28097809 Free PMC article.
-
3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment.Biomaterials. 2018 May;163:185-197. doi: 10.1016/j.biomaterials.2018.02.030. Epub 2018 Feb 13. Biomaterials. 2018. PMID: 29477032
-
TGFβ-specific T cells induced by a TGFβ-derived immune modulatory vaccine both directly and indirectly modulate the phenotype of tumor-associated macrophages and fibroblasts.J Immunother Cancer. 2024 Feb 27;12(2):e008405. doi: 10.1136/jitc-2023-008405. J Immunother Cancer. 2024. PMID: 38417917 Free PMC article.
-
Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity.Int J Mol Sci. 2019 Feb 5;20(3):676. doi: 10.3390/ijms20030676. Int J Mol Sci. 2019. PMID: 30764482 Free PMC article. Review.
-
Myeloid-derived suppressor cells and their role in pancreatic cancer.Cancer Gene Ther. 2017 Mar;24(3):100-105. doi: 10.1038/cgt.2016.65. Epub 2016 Dec 2. Cancer Gene Ther. 2017. PMID: 27910857 Review.
Cited by
-
The Cancer Microenvironment: Mechanical Challenges of the Metastatic Cascade.Front Bioeng Biotechnol. 2021 Feb 12;9:625859. doi: 10.3389/fbioe.2021.625859. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33644019 Free PMC article. Review.
-
3D Multicellular Tumor Spheroids in a Microfluidic Droplet System for Investigation of Drug Resistance.Polymers (Basel). 2022 Sep 8;14(18):3752. doi: 10.3390/polym14183752. Polymers (Basel). 2022. PMID: 36145898 Free PMC article.
-
Different Influences of Biotinylation and PEGylation on Cationic and Anionic Proteins for Spheroid Penetration and Intracellular Uptake to Cancer Cells.J Microbiol Biotechnol. 2022 Sep 28;32(9):1209-1216. doi: 10.4014/jmb.2207.07058. Epub 2022 Aug 29. J Microbiol Biotechnol. 2022. PMID: 36039388 Free PMC article.
-
Pancreatic Ductal Adenocarcinoma: Preclinical in vitro and ex vivo Models.Front Cell Dev Biol. 2021 Oct 22;9:741162. doi: 10.3389/fcell.2021.741162. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34746135 Free PMC article. Review.
-
Extracellular Vesicles and Resistance to Anticancer Drugs: A Tumor Skeleton Key for Unhinging Chemotherapies.Front Oncol. 2022 Jun 23;12:933675. doi: 10.3389/fonc.2022.933675. eCollection 2022. Front Oncol. 2022. PMID: 35814444 Free PMC article. Review.
References
-
- Chand S, O'Hayer K, Blanco FF, Winter JM, Brody JR. The Landscape of Pancreatic Cancer Therapeutic Resistance Mechanisms. Int J Biol Sci. 2016;12(3):273–82. Epub 2016/03/02. doi: 10.7150/ijbs.14951 . - DOI - PMC - PubMed
-
- Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–88. Epub 2007/11/28. doi: 10.1146/annurev.pathmechdis.3.121806.154305 . - DOI - PMC - PubMed
-
- Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol. 2013;4 Artn 210 doi: 10.3389/fphys.2013.00210 - DOI - PMC - PubMed
-
- Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167(2):e211–9. Epub 2009/09/22. doi: 10.1016/j.jss.2009.05.026 . - DOI - PubMed
-
- Helm O, Held-Feindt J, Grage-Griebenow E, Reiling N, Ungefroren H, Vogel I, et al. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer. 2014;135(4):843–61. Epub 2014/01/25. doi: 10.1002/ijc.28736 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

