Human cytomegalovirus IE1 downregulates Hes1 in neural progenitor cells as a potential E3 ubiquitin ligase
- PMID: 28750047
- PMCID: PMC5549770
- DOI: 10.1371/journal.ppat.1006542
Human cytomegalovirus IE1 downregulates Hes1 in neural progenitor cells as a potential E3 ubiquitin ligase
Abstract
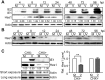
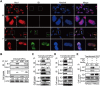
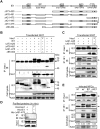
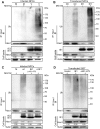
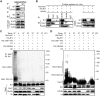
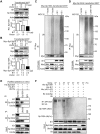
Congenital human cytomegalovirus (HCMV) infection is the leading cause of neurological disabilities in children worldwide, but the mechanisms underlying these disorders are far from well-defined. HCMV infection has been shown to dysregulate the Notch signaling pathway in human neural progenitor cells (NPCs). As an important downstream effector of Notch signaling, the transcriptional regulator Hairy and Enhancer of Split 1 (Hes1) is essential for governing NPC fate and fetal brain development. In the present study, we report that HCMV infection downregulates Hes1 protein levels in infected NPCs. The HCMV 72-kDa immediate-early 1 protein (IE1) is involved in Hes1 degradation by assembling a ubiquitination complex and promoting Hes1 ubiquitination as a potential E3 ubiquitin ligase, followed by proteasomal degradation of Hes1. Sp100A, an important component of PML nuclear bodies, is identified to be another target of IE1-mediated ubiquitination. A C-terminal acidic region in IE1, spanning amino acids 451 to 475, is required for IE1/Hes1 physical interaction and IE1-mediated Hes1 ubiquitination, but is dispensable for IE1/Sp100A interaction and ubiquitination. Our study suggests a novel mechanism linking downregulation of Hes1 protein to neurodevelopmental disorders caused by HCMV infection. Our findings also complement the current knowledge of herpesviruses by identifying IE1 as the first potential HCMV-encoded E3 ubiquitin ligase.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic Congenital Cytomegalovirus-Infection—Neonatal Morbidity and Mortality. Pediatr Infect Dis J. 1992;11(2):93–9. doi: 10.1097/00006454-199202000-00007 - DOI - PubMed
-
- Conboy TJ, Pass RF, Stagno S, Britt WJ, Alford CA, McFarland CE, et al. Intellectual development in school-aged children with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1986;77(6):801–6. . - PubMed
-
- Rosenthal LS, Fowler KB, Boppana SB, Britt WJ, Pass RF, Schmid SD, et al. Cytomegalovirus Shedding and Delayed Sensorineural Hearing Loss Results From Longitudinal Follow-up of Children With Congenital Infection. Pediatr Infect Dis J. 2009;28(6):515–20. doi: 10.1097/INF.0b013e318198c724 - DOI - PMC - PubMed
-
- Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, et al. Primary Cytomegalovirus-Infection in Pregnancy—Incidence, Transmission to Fetus, and Clinical Outcome. Jama-J Am Med Assoc. 1986;256(14):1904–8. - PubMed
-
- Li XJ, Liu XJ, Yang B, Fu YR, Zhao F, Shen ZZ, et al. Human Cytomegalovirus Infection Dysregulates the Localization and Stability of NICD1 and Jag1 in Neural Progenitor Cells. Journal of virology. 2015;89(13):6792–804. doi: 10.1128/JVI.00351-15 ; - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

