Bacterial diversity in Buruli ulcer skin lesions: Challenges in the clinical microbiome analysis of a skin disease
- PMID: 28750103
- PMCID: PMC5531519
- DOI: 10.1371/journal.pone.0181994
Bacterial diversity in Buruli ulcer skin lesions: Challenges in the clinical microbiome analysis of a skin disease
Abstract
Background: Buruli ulcer (BU) is an infectious disease caused by Mycobacterium ulcerans and considered the third most prevalent mycobacterial disease in humans. Secondary bacterial infections in open BU lesions are the main cause of pain, delayed healing and systemic illness, resulting in prolonged hospital stay. Thus, understanding the diversity of bacteria, termed the microbiome, in these open lesions is important for proper treatment. However, adequately studying the human microbiome in a clinical setting can prove difficult when investigating a neglected tropical skin disease due to its rarity and the setting.
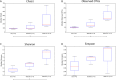
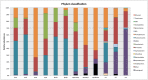
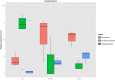
Methodology/principal findings: Using 16S rRNA sequencing, we determined the microbial composition of 5 BU lesions, 3 non-BU lesions and 3 healthy skin samples. Although no significant differences in diversity were found between BU and non-BU lesions, the former were characterized by an increase of Bacteroidetes compared to the non-BU wounds and the BU lesions also contained significantly more obligate anaerobes. With this molecular-based study, we were also able to detect bacteria that were missed by culture-based methods in previous BU studies.
Conclusions/significance: Our study suggests that BU may lead to changes in the skin bacterial community within the lesions. However, in order to determine if such changes hold true across all BU cases and are either a cause or consequence of a specific wound environment, further microbiome studies are necessary. Such skin microbiome analysis requires large sample sizes and lesions from the same body site in many patients, both of which can be difficult for a rare disease. Our study proposes a pipeline for such studies and highlights several drawbacks that must be considered if microbiome analysis is to be utilized for neglected tropical diseases.
Conflict of interest statement
Figures

References
-
- Walsh DS, Portaels F, Meyers WM. Buruli ulcer: Advances in understanding Mycobacterium ulcerans infection. Dermatol Clin. 2011;29: 1–8. doi: 10.1016/j.det.2010.09.006 - DOI - PubMed
-
- Organisation mondiale de la Santé. Traitement de l’infection à mycobacterium ulcerans (ulcère de Buruli): recommandations à l’intention des agents de santé. Organisation mondiale de la Santé; 2012;
-
- Kibadi K, Boelaert M, Fraga AG, Kayinua M, Longatto-Filho A, Minuku J-B, et al. Response to treatment in a prospective cohort of patients with large ulcerated lesions suspected to be Buruli Ulcer (Mycobacterium ulcerans disease). PLoS Negl Trop Dis. 2010;4: e736 doi: 10.1371/journal.pntd.0000736 - DOI - PMC - PubMed
-
- Barogui YT, Klis S, Bankolé HS, Sopoh GE, Mamo S, Baba-Moussa L, et al. Towards Rational Use of Antibiotics for Suspected Secondary Infections in Buruli Ulcer Patients. Small PLC, editor. PLoS Negl Trop Dis. Public Library of Science; 2013;7: e2010 doi: 10.1371/journal.pntd.0002010 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

