Cholesterol is required for stability and infectivity of influenza A and respiratory syncytial viruses
- PMID: 28750327
- PMCID: PMC5571833
- DOI: 10.1016/j.virol.2017.07.024
Cholesterol is required for stability and infectivity of influenza A and respiratory syncytial viruses
Abstract
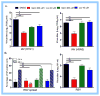
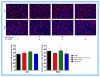
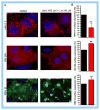
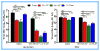
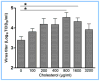
Cholesterol-rich lipid raft microdomains in the plasma membrane are considered to play a major role in the enveloped virus lifecycle. However, the functional role of cholesterol in assembly, infectivity and stability of respiratory RNA viruses is not fully understood. We previously reported that depletion of cellular cholesterol by cholesterol-reducing agents decreased production of human parainfluenza virus type 1 (hPIV1) particles by inhibiting virus assembly. In this study, we analyzed the role of cholesterol on influenza A virus (IAV) and respiratory syncytial virus (RSV) production. Unlike hPIV1, treatment of human airway cells with the agents did not decrease virus particle production. However, the released virions were less homogeneous in density and unstable. Addition of exogenous cholesterol to the released virions restored virus stability and infectivity. Collectively, these data indicate a critical role of cholesterol in maintaining IAV and RSV membrane structure that is essential for sustaining viral stability and infectivity.
Keywords: Antiviral therapy; Cholesterol; Lipid rafts; Respiratory viruses; Virus stability.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

