Label-free molecular imaging of the kidney
- PMID: 28750926
- PMCID: PMC6193761
- DOI: 10.1016/j.kint.2017.03.052
Label-free molecular imaging of the kidney
Abstract
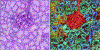
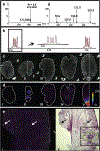
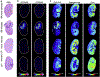
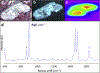
In this review, we will highlight technologies that enable scientists to study the molecular characteristics of tissues and/or cells without the need for antibodies or other labeling techniques. Specifically, we will focus on matrix-assisted laser desorption/ionization imaging mass spectrometry, infrared spectroscopy, and Raman spectroscopy.
Keywords: advanced-glycation end products; cancer; fibrosis; inflammation; renal biopsy; renal pathology.
Copyright © 2017 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE
All the authors declared no competing interests.
Figures







References
-
- Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem. 1997;69:4751–4760. - PubMed
-
- McDonnell LA, Heeren RMA. Imaging mass spectrometry. Mass Spectrom Rev. 2007;26:606–643. - PubMed
-
- Spengler B Mass spectrometry imaging of biomolecular information. Anal Chem. 2015;87:64–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

