Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers
- PMID: 28751442
- PMCID: PMC5771850
- DOI: 10.1158/1078-0432.CCR-16-0037
Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers
Abstract
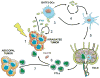
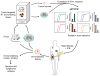
The first evidence that radiotherapy enhances the efficacy of immune checkpoint blockers (ICB) was obtained a dozen years ago in a mouse model of metastatic carcinoma refractory to anti-CTLA-4 treatment. At the time, ICBs had just entered clinical testing, an endeavor that culminated in 2011 with the approval of the first anti-CTLA-4 antibody for use in metastatic melanoma patients (ipilimumab). Thereafter, some patients progressing on ipilimumab showed systemic responses only upon receiving radiation to one lesion, confirming clinically the proimmunogenic effects of radiation. Preclinical data demonstrate that multiple immunomodulators synergize with radiotherapy to cause the regression of irradiated tumors and, less often, nonirradiated metastases. However, the impact of dose and fractionation on the immunostimulatory potential of radiotherapy has not been thoroughly investigated. This issue is extremely relevant given the growing number of clinical trials testing the ability of radiotherapy to increase the efficacy of ICBs. Recent data demonstrate that the recruitment of dendritic cells to neoplastic lesions (and hence the priming of tumor-specific CD8+ T cells) is highly dependent on radiotherapy dose and fractionation through a mechanism that involves the accumulation of double-stranded DNA in the cytoplasm of cancer cells and consequent type I IFN release. The molecular links between the cellular response to radiotherapy and type I IFN secretion are just being uncovered. Here, we discuss the rationale for an optimized use of radiotherapy as well as candidate biomarkers that may predict clinical responses to radiotherapy combined with ICBs. Clin Cancer Res; 24(2); 259-65. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Ledford H. Melanoma drug wins US approval. Nature. 2011;471:561. - PubMed
-
- Postow MA, Callahan MK, Wolchok JD. The antitumor immunity of ipilimumab: (T-cell) memories to last a lifetime? Clin Cancer Res. 2012;18:1821–3. - PubMed
-
- Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1:1325–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

