Optogenetic Central Amygdala Stimulation Intensifies and Narrows Motivation for Cocaine
- PMID: 28751460
- PMCID: PMC5577851
- DOI: 10.1523/JNEUROSCI.3141-16.2017
Optogenetic Central Amygdala Stimulation Intensifies and Narrows Motivation for Cocaine
Abstract
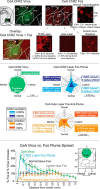
Addiction is often characterized by intense motivation for a drug, which may be narrowly focused at the expense of other rewards. Here, we examined the role of amygdala-related circuitry in the amplification and narrowing of motivation focus for intravenous cocaine. We paired optogenetic channelrhodopsin (ChR2) stimulation in either central nucleus of amygdala (CeA) or basolateral amygdala (BLA) of female rats with one particular nose-poke porthole option for earning cocaine infusions (0.3 mg/kg, i.v.). A second alternative porthole earned identical cocaine but without ChR2 stimulation. Consequently, CeA rats quickly came to pursue their CeA ChR2-paired cocaine option intensely and exclusively, elevating cocaine intake while ignoring their alternative cocaine alone option. By comparison, BLA ChR2 pairing failed to enhance cocaine motivation. CeA rats also emitted consummatory bites toward their laser-paired porthole, suggesting that higher incentive salience made that cue more attractive. A separate progressive ratio test of incentive motivation confirmed that CeA ChR2 amplified rats' motivation, raising their breakpoint effort price for cocaine by 10-fold. However, CeA ChR2 laser on its own lacked any reinforcement value: laser by itself was never self-stimulated, not even by the same rats in which it amplified motivation for cocaine. Conversely, CeA inhibition by muscimol/baclofen microinjections prevented acquisition of cocaine self-administration and laser preference, whereas CeA inhibition by optogenetic halorhodopsin suppressed cocaine intake, indicating that CeA circuitry is needed for ordinary cocaine motivation. We conclude that CeA ChR2 excitation paired with a cocaine option specifically focuses and amplifies motivation to produce intense pursuit and consumption focused on that single target.SIGNIFICANCE STATEMENT In addiction, intense incentive motivation often becomes narrowly focused on a particular drug of abuse. Here we show that pairing central nucleus of amygdala (CeA) optogenetic stimulation with one option for earning intravenous cocaine makes that option almost the exclusive focus of intense pursuit and consumption. CeA stimulation also elevated the effort cost rats were willing to pay for cocaine and made associated cues become intensely attractive. However, we also show that CeA laser had no reinforcing properties at all when given alone for the same rats. Therefore, CeA laser pairing makes its associated cocaine option and cues become powerfully attractive in a nearly addictive fashion.
Keywords: addiction; amygdala; intravenous self-administration; motivation; reward.
Copyright © 2017 the authors 0270-6474/17/378330-19$15.00/0.
Figures









Similar articles
-
Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another.J Neurosci. 2014 Dec 10;34(50):16567-80. doi: 10.1523/JNEUROSCI.2013-14.2014. J Neurosci. 2014. PMID: 25505310 Free PMC article.
-
Optogenetic activation of the central amygdala generates addiction-like preference for reward.Eur J Neurosci. 2019 Aug;50(3):2086-2100. doi: 10.1111/ejn.13967. Epub 2018 Jun 15. Eur J Neurosci. 2019. PMID: 29797474
-
Optogenetic Activation of the Basolateral Amygdala Promotes Both Appetitive Conditioning and the Instrumental Pursuit of Reward Cues.J Neurosci. 2020 Feb 19;40(8):1732-1743. doi: 10.1523/JNEUROSCI.2196-19.2020. Epub 2020 Jan 17. J Neurosci. 2020. PMID: 31953370 Free PMC article.
-
Incentive motivation: 'wanting' roles of central amygdala circuitry.Behav Brain Res. 2021 Aug 6;411:113376. doi: 10.1016/j.bbr.2021.113376. Epub 2021 May 20. Behav Brain Res. 2021. PMID: 34023307 Free PMC article. Review.
-
Dyadic social interaction as an alternative reward to cocaine.Front Psychiatry. 2013 Sep 12;4:100. doi: 10.3389/fpsyt.2013.00100. Front Psychiatry. 2013. PMID: 24062696 Free PMC article. Review.
Cited by
-
Optogenetic self-stimulation in the nucleus accumbens: D1 reward versus D2 ambivalence.PLoS One. 2018 Nov 29;13(11):e0207694. doi: 10.1371/journal.pone.0207694. eCollection 2018. PLoS One. 2018. PMID: 30496206 Free PMC article.
-
Molecular fingerprints in the hippocampus of alcohol seeking during withdrawal.bioRxiv [Preprint]. 2023 Aug 26:2023.08.24.554622. doi: 10.1101/2023.08.24.554622. bioRxiv. 2023. PMID: 37662388 Free PMC article. Preprint.
-
Dynamic regulation of the extracellular matrix in reward memory processes: a question of time.Front Cell Neurosci. 2023 Jun 16;17:1208974. doi: 10.3389/fncel.2023.1208974. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37396928 Free PMC article. Review.
-
A Systematic Review of Structural and Functional MRI Studies Investigating Social Networking Site Use.Brain Sci. 2023 May 11;13(5):787. doi: 10.3390/brainsci13050787. Brain Sci. 2023. PMID: 37239257 Free PMC article. Review.
-
Ventromedial frontoinsular connectivity is associated with long-term smoking behavior change in aging.Imaging Neurosci (Camb). 2024 May 9;2:imag-2-00142. doi: 10.1162/imag_a_00142. eCollection 2024. Imaging Neurosci (Camb). 2024. PMID: 40800324 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
