Parkinson disease-associated LRRK2 G2019S transgene disrupts marrow myelopoiesis and peripheral Th17 response
- PMID: 28751472
- PMCID: PMC5597519
- DOI: 10.1189/jlb.1A0417-147RR
Parkinson disease-associated LRRK2 G2019S transgene disrupts marrow myelopoiesis and peripheral Th17 response
Abstract
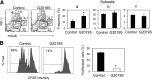
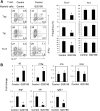
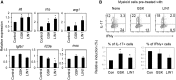
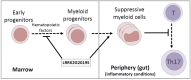
Parkinson's disease (PD) is a neurodegenerative disease, whereas Crohn's disease is an inflammatory bowel disease. Interestingly, polymorphisms in the LRRK2 gene have been identified as risk factors for both diseases. LRRK2 G2019S is the most prevalent mutation found in PD. To gain insights into the role of the LRRK2 G2019S gene on the development and activation of the immune system in the brain-gut axis, we investigated the effect of LRRK2 G2019S on bone marrow myeloid progenitors and myeloid cell function in the periphery. We used bacterial artificial chromosome transgenic rats harboring the human LRRK2 G2019S gene. LRRK2 G2019S transgene decreased the numbers of monocytic and granulocytic progenitors in the bone marrow. However, the numbers of peripheral, immature myeloid cells with suppressive activity were increased in the gut and blood circulation of LRRK2 G2019S compared with control rats in various acute and chronic inflammatory responses. In inflammatory conditions, Th17 cell activity was suppressed, but tissue-associated phylum Bacteroidetes was abnormally increased in the intestine of LRRK2 G2019S rats. The abnormally expanded myeloid cells because of the LRRK2 G2019S gene were highly suppressive on Th17 cell differentiation. Moreover, we found that inhibition of LRRK2 kinase affects myeloid progenitors and myeloid cell differentiation. Taken together, the results indicate that abnormal LRRK2 activity can alter bone marrow myelopoiesis, peripheral myeloid cell differentiation, and intestinal immune homeostasis. These findings may have ramifications in immune and inflammatory responses in patients with LRRK2 abnormalities.
Keywords: Crohn; T cells; inflammation; intestine; myeloid cells.
© Society for Leukocyte Biology.
Figures



References
-
- Nichols W. C., Pankratz N., Hernandez D., Paisán-Ruíz C., Jain S., Halter C. A., Michaels V. E., Reed T., Rudolph A., Shults C. W., Singleton A., Foroud T.; Parkinson Study Group-PROGENI investigators (2005) Genetic screening for a single common LRRK2 mutation in familial Parkinson’s disease. Lancet 365, 410–412. - PubMed
-
- Di Fonzo A., Rohé C. F., Ferreira J., Chien H. F., Vacca L., Stocchi F., Guedes L., Fabrizio E., Manfredi M., Vanacore N., Goldwurm S., Breedveld G., Sampaio C., Meco G., Barbosa E., Oostra B. A., Bonifati V.; Italian Parkinson Genetics Network (2005) A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson’s disease. Lancet 365, 412–415. - PubMed
-
- Gilks W. P., Abou-Sleiman P. M., Gandhi S., Jain S., Singleton A., Lees A. J., Shaw K., Bhatia K. P., Bonifati V., Quinn N. P., Lynch J., Healy D. G., Holton J. L., Revesz T., Wood N. W. (2005) A common LRRK2 mutation in idiopathic Parkinson’s disease. Lancet 365, 415–416. - PubMed
-
- Greggio E. (2012) Role of LRRK2 kinase activity in the pathogenesis of Parkinson’s disease. Biochem. Soc. Trans. 40, 1058–1062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

