Downregulation of Cavin-1 Expression via Increasing Caveolin-1 Degradation Prompts the Proliferation and Migration of Vascular Smooth Muscle Cells in Balloon Injury-Induced Neointimal Hyperplasia
- PMID: 28751541
- PMCID: PMC5586430
- DOI: 10.1161/JAHA.117.005754
Downregulation of Cavin-1 Expression via Increasing Caveolin-1 Degradation Prompts the Proliferation and Migration of Vascular Smooth Muscle Cells in Balloon Injury-Induced Neointimal Hyperplasia
Abstract
Background: Percutaneous coronary intervention has been widely used in the treatment of ischemic heart disease, but vascular restenosis is a main limitation of percutaneous coronary intervention. Our previous work reported that caveolin-1 had a key functional role in intimal hyperplasia, whereas whether Cavin-1 (another important caveolae-related protein) was involved is still unknown. Therefore, we will investigate the effect of Cavin-1 on neointimal formation.
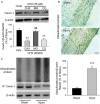
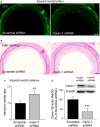
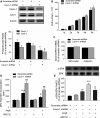
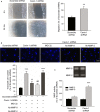
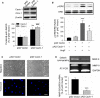
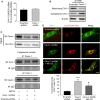
Methods and results: Balloon injury markedly reduced Cavin-1 protein and enhanced ubiquitin protein expression accompanied with neointimal hyperplasia in injured carotid arteries, whereas Cavin-1 mRNA had no change. In cultured vascular smooth muscle cells (VSMCs), Cavin-1 was downregulated after inhibition of protein synthesis by cycloheximide, which was distinctly prevented by pretreatment with proteasome inhibitor MG132 but not by lysosomal inhibitor chloroquine, suggesting that proteasomal degradation resulted in Cavin-1 downregulation. Knockdown of Cavin-1 by local injection of Cavin-1 short hairpin RNA (shRNA) into balloon-injured carotid arteries in vivo promoted neointimal formation. In addition, inhibition or overexpression of Cavin-1 in cultured VSMCs in vitro prompted or suppressed VSMC proliferation and migration via increasing or decreasing extracellular signal-regulated kinase phosphorylation and matrix-degrading metalloproteinases-9 activity, respectively. However, under basic conditions, the effect of Cavin-1 on VSMC migration was stronger than on proliferation. Moreover, our results indicated that Cavin-1 regulated caveolin-1 expression via lysosomal degradation pathway.
Conclusions: Our study revealed the role and the mechanisms of Cavin-1 downregulation in neointimal formation by promoting VSMC proliferation, migration, and synchronously enhancing caveolin-1 lysosomal degradation. Cavin-1 may be a potential therapeutic target for the treatment of postinjury vascular remodeling.
Keywords: Cavin‐1; caveolin‐1; migration; neointimal hyperplasia; polymerase I and transcript release factor; proliferation; vascular smooth muscle.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures

References
-
- Nagaraja V, Ooi SY, Nolan J, Large A, De BM, Ludman P, Bagur R, Curzen N, Matsukage T, Yoshimachi F, Kwok CS, Berry C, Mamas MA. Impact of incomplete percutaneous revascularization in patients with multivessel coronary artery disease: a systematic review and meta‐analysis. J Am Heart Assoc. 2016;5:e004598 DOI: - DOI - PMC - PubMed
-
- Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in‐stent restenosis. J Am Coll Cardiol. 2014;63:2659–2673. - PubMed
-
- Misumida N, Aoi S, Saeed M, Ota T, Eda T, Umeda H, Kanei Y. The role of angiographic follow‐up after percutaneous coronary intervention. Int J Cardiol. 2016;222:911–920. - PubMed
-
- Siontis GC, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Perez‐Vizcayno MJ, Byrne RA, Kastrati A, Meier B, Salanti G, Juni P, Windecker S. Percutaneous coronary interventional strategies for treatment of in‐stent restenosis: a network meta‐analysis. Lancet. 2015;386:655–664. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

