ADPase CD39 Fused to Glycoprotein VI-Fc Boosts Local Antithrombotic Effects at Vascular Lesions
- PMID: 28751543
- PMCID: PMC5586441
- DOI: 10.1161/JAHA.117.005991
ADPase CD39 Fused to Glycoprotein VI-Fc Boosts Local Antithrombotic Effects at Vascular Lesions
Abstract
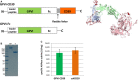
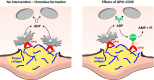
Background: GPVI (Glycoprotein VI) is the essential platelet collagen receptor in atherothrombosis. Dimeric GPVI-Fc (Revacept) binds to GPVI binding sites on plaque collagen. As expected, it did not increase bleeding in clinical studies. GPVI-Fc is a potent inhibitor of atherosclerotic plaque-induced platelet aggregation at high shear flow, but its inhibition at low shear flow is limited. We sought to increase the platelet inhibitory potential by fusing GPVI-Fc to the ectonucleotidase CD39 (fusion protein GPVI-CD39), which inhibits local ADP accumulation at vascular plaques, and thus to create a lesion-directed dual antiplatelet therapy that is expected to lack systemic bleeding risks.
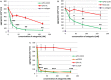
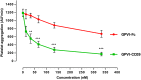
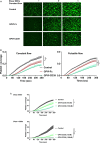
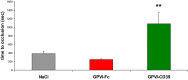
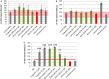
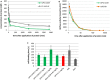
Methods and results: GPVI-CD39 effectively stimulated local ADP degradation and, compared with GPVI-Fc alone, led to significantly increased inhibition of ADP-, collagen-, and human plaque-induced platelet aggregation in Multiplate aggregometry and plaque-induced platelet thrombus formation under arterial flow conditions. GPVI-CD39 did not increase bleeding time in an in vitro assay simulating primary hemostasis. In a mouse model of ferric chloride-induced arterial thrombosis, GPVI-CD39 effectively delayed vascular thrombosis but did not increase tail bleeding time in vivo.
Conclusions: GPVI-CD39 is a novel approach to increase local antithrombotic activity at sites of atherosclerotic plaque rupture or injury. It enhances GPVI-Fc-mediated platelet inhibition and presents a potentially effective and safe molecule for the treatment of acute atherothrombotic events, with a favorable risk-benefit ratio.
Keywords: glycoprotein; platelet; thrombosis.
© 2017 The Authors and AdvanceCor GmbH. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures
Similar articles
-
Recombinant GPVI-Fc added to single or dual antiplatelet therapy in vitro prevents plaque-induced platelet thrombus formation.Thromb Haemost. 2017 Aug 1;117(8):1651-1659. doi: 10.1160/TH16-11-0856. Epub 2017 Jun 1. Thromb Haemost. 2017. PMID: 28569920
-
Trowaglerix Venom Polypeptides As a Novel Antithrombotic Agent by Targeting Immunoglobulin-Like Domains of Glycoprotein VI in Platelet.Arterioscler Thromb Vasc Biol. 2017 Jul;37(7):1307-1314. doi: 10.1161/ATVBAHA.116.308604. Epub 2017 Jun 8. Arterioscler Thromb Vasc Biol. 2017. PMID: 28596377
-
Relative antithrombotic effect of soluble GPVI dimer compared with anti-GPVI antibodies in mice.Blood. 2005 Feb 15;105(4):1492-9. doi: 10.1182/blood-2004-06-2391. Epub 2004 Oct 26. Blood. 2005. PMID: 15507524
-
Glycoprotein VI - novel target in antiplatelet medication.Pharmacol Ther. 2021 Jan;217:107630. doi: 10.1016/j.pharmthera.2020.107630. Epub 2020 Jul 16. Pharmacol Ther. 2021. PMID: 32681846 Review.
-
GPVI inhibition: Advancing antithrombotic therapy in cardiovascular disease.Eur Heart J Cardiovasc Pharmacother. 2024 Aug 14;10(5):465-473. doi: 10.1093/ehjcvp/pvae018. Eur Heart J Cardiovasc Pharmacother. 2024. PMID: 38453424 Free PMC article. Review.
Cited by
-
Systematic Reviews of the Literature Are Not Always Either Useful Or the Best Way To Add To Science.EJVES Vasc Forum. 2021 Nov 16;54:2-6. doi: 10.1016/j.ejvsvf.2021.10.021. eCollection 2022. EJVES Vasc Forum. 2021. PMID: 34917997 Free PMC article. Review.
-
A review of pig liver xenotransplantation: Current problems and recent progress.Xenotransplantation. 2019 May;26(3):e12497. doi: 10.1111/xen.12497. Epub 2019 Feb 15. Xenotransplantation. 2019. PMID: 30767272 Free PMC article. Review.
-
Characterization of GPVI- or GPVI-CD39-Coated Nanoparticles and Their Impact on In Vitro Thrombus Formation.Int J Mol Sci. 2021 Dec 21;23(1):11. doi: 10.3390/ijms23010011. Int J Mol Sci. 2021. PMID: 35008437 Free PMC article.
-
Factors Associated with Platelet Activation-Recent Pharmaceutical Approaches.Int J Mol Sci. 2022 Mar 18;23(6):3301. doi: 10.3390/ijms23063301. Int J Mol Sci. 2022. PMID: 35328719 Free PMC article. Review.
-
Ectonucleoside Triphosphate Diphosphohydrolase-1/CD39 Affects the Response to ADP of Female Rat Platelets.Front Pharmacol. 2020 Jan 31;10:1689. doi: 10.3389/fphar.2019.01689. eCollection 2019. Front Pharmacol. 2020. PMID: 32082171 Free PMC article.
References
-
- Mackay J, Mensah G. The Atlas of heart disease and stroke. World Health Organisation: Geneva, Switzerland, 2004. Available at: http://www.who.int/cardiovascular_diseases/resources/atlas/en/. Accessed July 4, 2017.
-
- Marsh JD, Keyrouz SG. Stroke prevention and treatment. J Am Coll Cardiol. 2010;56:683–691. - PubMed
-
- Stoll G, Kleinschnitz C, Nieswandt B. The role of glycoprotein Ibalpha and von Willebrand factor interaction in stroke development. Hamost. 2010;30:136–138. - PubMed
-
- Penz S, Reininger AJ, Brandl R, Goyal P, Rabie T, Bernlochner I, Rother E, Goetz C, Engelmann B, Smethurst PA, Ouwehand WH, Farndale R, Nieswandt B, Siess W. Human atheromatous plaques stimulate thrombus formation by activating platelet glycoprotein VI. Faseb J. 2005;19:898–909. - PubMed
-
- Reininger AJ, Bernlochner I, Penz SM, Ravanat C, Smethurst P, Farndale RW, Gachet C, Brandl R, Siess W. A 2‐step mechanism of arterial thrombus formation induced by human atherosclerotic plaques. J Am Coll Cardiol. 2010;55:1147–1158. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

