Angiotensin II Type 1 Receptor-Associated Protein Regulates Kidney Aging and Lifespan Independent of Angiotensin
- PMID: 28751545
- PMCID: PMC5586453
- DOI: 10.1161/JAHA.117.006120
Angiotensin II Type 1 Receptor-Associated Protein Regulates Kidney Aging and Lifespan Independent of Angiotensin
Abstract
Background: The kidney is easily affected by aging-associated changes, including glomerulosclerosis, tubular atrophy, and interstitial fibrosis. Particularly, renal tubulointerstitial fibrosis is a final common pathway in most forms of progressive renal disease. Angiotensin II type 1 receptor (AT1R)-associated protein (ATRAP), which was originally identified as a molecule that binds to AT1R, is highly expressed in the kidney. Previously, we have shown that ATRAP suppresses hyperactivation of AT1R signaling, but does not affect physiological AT1R signaling.
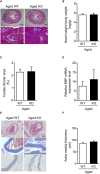
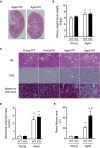
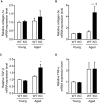
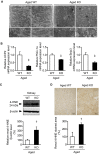
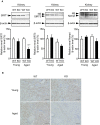
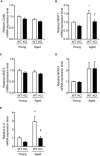
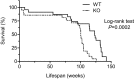
Methods and results: We hypothesized that ATRAP has a novel functional role in the physiological age-degenerative process, independent of modulation of AT1R signaling. ATRAP-knockout mice were used to study the functional involvement of ATRAP in the aging. ATRAP-knockout mice exhibit a normal age-associated appearance without any evident alterations in physiological parameters, including blood pressure and cardiovascular and metabolic phenotypes. However, in ATRAP-knockout mice compared with wild-type mice, the following takes place: (1) age-associated renal function decline and tubulointerstitial fibrosis are more enhanced; (2) renal tubular mitochondrial abnormalities and subsequent increases in the production of reactive oxygen species are more advanced; and (3) life span is 18.4% shorter (median life span, 100.4 versus 123.1 weeks). As a key mechanism, age-related pathological changes in the kidney of ATRAP-knockout mice correlated with decreased expression of the prosurvival gene, Sirtuin1. On the other hand, chronic angiotensin II infusion did not affect renal sirtuin1 expression in wild-type mice.
Conclusions: These results indicate that ATRAP plays an important role in inhibiting kidney aging, possibly through sirtuin1-mediated mechanism independent of blocking AT1R signaling, and further protecting normal life span.
Keywords: aging; chronic kidney disease; fibrosis; renin angiotensin system.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures


Similar articles
-
An angiotensin II type 1 receptor binding molecule has a critical role in hypertension in a chronic kidney disease model.Kidney Int. 2017 May;91(5):1115-1125. doi: 10.1016/j.kint.2016.10.035. Epub 2017 Jan 10. Kidney Int. 2017. PMID: 28081856
-
Angiotensin II Type 1 Receptor Binding Molecule ATRAP as a Possible Modulator of Renal Sodium Handling and Blood Pressure in Pathophysiology.Curr Med Chem. 2015;22(28):3210-6. doi: 10.2174/0929867322666150821095036. Curr Med Chem. 2015. PMID: 26295465 Review.
-
Renal tubule angiotensin II type 1 receptor-associated protein promotes natriuresis and inhibits salt-sensitive blood pressure elevation.J Am Heart Assoc. 2015 Mar 19;4(3):e001594. doi: 10.1161/JAHA.114.001594. J Am Heart Assoc. 2015. PMID: 25792129 Free PMC article.
-
Intrarenal suppression of angiotensin II type 1 receptor binding molecule in angiotensin II-infused mice.Am J Physiol Renal Physiol. 2010 Nov;299(5):F991-F1003. doi: 10.1152/ajprenal.00738.2009. Epub 2010 Aug 25. Am J Physiol Renal Physiol. 2010. PMID: 20739392
-
The pathophysiological role of angiotensin receptor-binding protein in hypertension and kidney diseases: Oshima Award Address 2019.Clin Exp Nephrol. 2020 Apr;24(4):289-294. doi: 10.1007/s10157-020-01861-4. Epub 2020 Feb 29. Clin Exp Nephrol. 2020. PMID: 32112267 Free PMC article. Review.
Cited by
-
Oxidative stress and senescence in aging kidneys: the protective role of SIRT1.EXCLI J. 2024 Aug 27;23:1030-1067. doi: 10.17179/excli2024-7519. eCollection 2024. EXCLI J. 2024. PMID: 39391060 Free PMC article. Review.
-
Age‑related changes in mineralocorticoid receptors in rat hearts.Mol Med Rep. 2020 Sep;22(3):1859-1867. doi: 10.3892/mmr.2020.11260. Epub 2020 Jun 19. Mol Med Rep. 2020. PMID: 32582979 Free PMC article.
-
Angiotensin II type-1 receptor-associated protein interacts with transferrin receptor-1 and promotes its internalization.Sci Rep. 2022 Oct 17;12(1):17376. doi: 10.1038/s41598-022-22343-5. Sci Rep. 2022. PMID: 36253401 Free PMC article.
-
Effects of ATRAP in Renal Proximal Tubules on Angiotensin-Dependent Hypertension.J Am Heart Assoc. 2019 Apr 16;8(8):e012395. doi: 10.1161/JAHA.119.012395. J Am Heart Assoc. 2019. PMID: 30977419 Free PMC article.
-
Recent Research Advances in Renin-Angiotensin-Aldosterone System Receptors.Curr Hypertens Rep. 2020 Feb 29;22(3):22. doi: 10.1007/s11906-020-1028-6. Curr Hypertens Rep. 2020. PMID: 32114685 Review.
References
-
- Finch CE, Tanzi RE. Genetics of aging. Science. 1997;278:407–411. - PubMed
-
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. - PubMed
-
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life‐span extension by calorie restriction in Saccharomyces cerevisiae . Science. 2000;289:2126–2128. - PubMed
-
- Kuro‐o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki‐Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

