Impact of prior therapy on the efficacy and safety of oral ixazomib-lenalidomide-dexamethasone vs. placebo-lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma in TOURMALINE-MM1
- PMID: 28751562
- PMCID: PMC5622861
- DOI: 10.3324/haematol.2017.170118
Impact of prior therapy on the efficacy and safety of oral ixazomib-lenalidomide-dexamethasone vs. placebo-lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma in TOURMALINE-MM1
Abstract
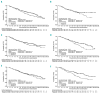
Prior treatment exposure in patients with relapsed/refractory multiple myeloma may affect outcomes with subsequent therapies. We analyzed efficacy and safety according to prior treatment in the phase 3 TOURMALINE-MM1 study of ixazomib-lenalidomide-dexamethasone (ixazomib-Rd) versus placebo-Rd. Patients with relapsed/refractory multiple myeloma received ixazomib-Rd or placebo-Rd. Efficacy and safety were evaluated in subgroups defined according to type (proteasome inhibitor [PI] and immunomodulatory drug) and number (1 vs. 2 or 3) of prior therapies received. Of 722 patients, 503 (70%) had received a prior PI, and 397 (55%) prior lenalidomide/thalidomide; 425 patients had received 1 prior therapy, and 297 received 2 or 3 prior therapies. At a median follow up of ~15 months, PFS was prolonged with ixazomib-Rd vs. placebo-Rd regardless of type of prior therapy received; HR 0.739 and 0.749 in PI-exposed and -naïve patients, HR 0.744 and 0.700 in immunomodulatory-drug-exposed and -naïve patients, respectively. PFS benefit with ixazomib-Rd vs. placebo-Rd appeared greater in patients with 2 or 3 prior therapies (HR 0.58) and in those with 1 prior therapy without prior transplant (HR 0.60) versus those with 1 prior therapy and transplant (HR 1.23). Across all subgroups, toxicity was consistent with that seen in the intent-to-treat population. In patients with relapsed/refractory multiple myeloma, ixazomib-Rd was associated with a consistent clinical benefit vs. placebo-Rd regardless of prior treatment with bortezomib or immunomodulatory drugs. Patients with 2 or 3 prior therapies, or 1 prior therapy without transplant seemed to have greater benefit than patients with 1 prior therapy and transplant. TOURMALINE-MM1 registered at clinicaltrials.gov identifier: 01564537.
Trial registration: ClinicalTrials.gov NCT01564537.
Copyright© 2017 Ferrata Storti Foundation.
Figures


Similar articles
-
Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China Continuation study.J Hematol Oncol. 2017 Jul 6;10(1):137. doi: 10.1186/s13045-017-0501-4. J Hematol Oncol. 2017. PMID: 28683766 Free PMC article. Clinical Trial.
-
Final Overall Survival Analysis of the TOURMALINE-MM1 Phase III Trial of Ixazomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma.J Clin Oncol. 2021 Aug 1;39(22):2430-2442. doi: 10.1200/JCO.21.00972. Epub 2021 Jun 11. J Clin Oncol. 2021. PMID: 34111952 Clinical Trial.
-
Healthcare resource utilization with ixazomib or placebo plus lenalidomide-dexamethasone in the randomized, double-blind, phase 3 TOURMALINE-MM1 study in relapsed/refractory multiple myeloma.J Med Econ. 2018 Aug;21(8):793-798. doi: 10.1080/13696998.2018.1474745. Epub 2018 May 29. J Med Econ. 2018. PMID: 29741409 Clinical Trial.
-
Ixazomib - the first oral proteasome inhibitor.Leuk Lymphoma. 2019 Mar;60(3):610-618. doi: 10.1080/10428194.2018.1523398. Epub 2019 Jan 7. Leuk Lymphoma. 2019. PMID: 30614337 Review.
-
Ixazomib: A Review in Relapsed and/or Refractory Multiple Myeloma.Target Oncol. 2017 Aug;12(4):535-542. doi: 10.1007/s11523-017-0504-7. Target Oncol. 2017. PMID: 28660423 Review.
Cited by
-
Cardiovascular Complications of Multiple Myeloma Treatment: Evaluation, Management, and Prevention.Curr Treat Options Cardiovasc Med. 2018 Mar 6;20(3):19. doi: 10.1007/s11936-018-0618-y. Curr Treat Options Cardiovasc Med. 2018. PMID: 29508087 Review.
-
The proteasome as a druggable target with multiple therapeutic potentialities: Cutting and non-cutting edges.Pharmacol Ther. 2020 Sep;213:107579. doi: 10.1016/j.pharmthera.2020.107579. Epub 2020 May 19. Pharmacol Ther. 2020. PMID: 32442437 Free PMC article.
-
Melphalan and Exportin 1 Inhibitors Exert Synergistic Antitumor Effects in Preclinical Models of Human Multiple Myeloma.Cancer Res. 2020 Dec 1;80(23):5344-5354. doi: 10.1158/0008-5472.CAN-19-0677. Epub 2020 Oct 6. Cancer Res. 2020. PMID: 33023948 Free PMC article.
-
c-MYC expression and maturity phenotypes are associated with outcome benefit from addition of ixazomib to lenalidomide-dexamethasone in myeloma.Eur J Haematol. 2020 Jul;105(1):35-46. doi: 10.1111/ejh.13405. Epub 2020 Apr 15. Eur J Haematol. 2020. PMID: 32145111 Free PMC article.
-
Mass Spectrometry-Based Method Targeting Ig Variable Regions for Assessment of Minimal Residual Disease in Multiple Myeloma.J Mol Diagn. 2020 Jul;22(7):901-911. doi: 10.1016/j.jmoldx.2020.04.002. Epub 2020 Apr 14. J Mol Diagn. 2020. PMID: 32302778 Free PMC article.
References
-
- Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111(5):2521–2526. - PubMed
-
- Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia. 2009;23(6):1152–1157. - PubMed
-
- Kristinsson SY, Landgren O, Dickman PW, Derolf AR, Bjorkholm M. Patterns of survival in multiple myeloma: a population-based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007;25(15):1993–1999. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

