Caregiver burden in schizophrenia following paliperidone palmitate long acting injectables treatment: pooled analysis of two double-blind randomized phase three studies
- PMID: 28751663
- PMCID: PMC5532271
- DOI: 10.1038/s41537-017-0025-5
Caregiver burden in schizophrenia following paliperidone palmitate long acting injectables treatment: pooled analysis of two double-blind randomized phase three studies
Abstract
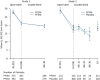
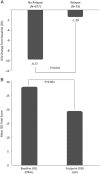
The pooled analysis of two double-blind, randomized, multicenter, phase-3 studies evaluated predictors of improvement or worsening of schizophrenia-related caregiver burden following paliperidone palmitate long-acting injectables (1-monthly [PP1M] and 3-monthly [PP3M]) treatment. Caregivers were offered to complete the involvement evaluation questionnaire (involvement evaluation questionnaire; 31-item scale). Total, 1498 caregivers (intent-to-treat open-label analysis set, n = 1497; mean [SD] age: 51.5 [13.02] years, 27 countries) were included: 49% were parents and >50% caregivers spent >32 hours/week in caregiving. Majority of caregivers with considerable burden (n = 1405; mean [SD] baseline involvement evaluation questionnaire scores: 28.4 [15.07]) improved significantly from baseline to end-of-study (n = 756; mean [SD] change from open-label baseline to double-blind endpoint in long-acting injectable scores:-8.9 [14.73]); most improvements were seen in urging followed by worrying, tension, and supervision domains (mean [SD] change from open-label baseline to double-blind endpoint in involvement evaluation questionnaire scores, urging: -3.7 [6.45]; worrying:-2.6 [5.11]; tension:-2.3 [4.84]; supervision: -1.3 [3.69]). Improvements significantly correlated with relapse status, patient age, and age of diagnosis (p < 0.001) while long-acting injectable use at baseline, number, and duration of prior psychiatric hospitalizations (<24 months) had no significant correlation. Caregiver burden was significantly improved for patients on prior oral antipsychotics post-switching to long-acting injectable, with less impact on leisure days and hours spent in caregiving (p < 0.001). Family members of patients with schizophrenia experience considerable caregiver burden. Switching from oral antipsychotic to long-acting injectable can provide meaningful and significant improvement in caregiver burden.
Drug formulation: EASING THE TOLL OF CAREGIVING: Switching from oral to long-acting injectable antipsychotic medication improves overall caregiver burden. The physical, emotional and financial toll of providing care for patients with schizophrenia is often underestimated. Poor adherence to conventional oral antipsychotics is a major cause of symptomatic relapse in patients and of stress for carers. Srihari Gopal and colleagues at Janssen Pharmaceuticals have pooled data from two large studies involving 1498 caregivers across 27 countries. They found that administration of either 1- or 3-monthly long-acting injectable antipsychotics not only eased the burden of daily dosing and patient compliance, but also had a positive impact on the stress conditions of caregivers. Using the Involvement Evaluation Questionnaire to measure caregiver burden, the authors showed that the switch in drug formulation decreased the need to urge patients to self-care and the hours spent caregiving.
Conflict of interest statement
All authors are employees of Janssen Research & Development and hold company stocks.
Figures
References
-
- Desai PR, et al. Estimating the direct and indirect costs for community-dwelling patients with schizophrenia. J Pharm Health Serv Res. 2013;4:187–194. doi: 10.1111/jphs.12027. - DOI
-
- Invega Trinza Prescribing Information (2015). http://www.janssencns.com/shared/product/invegatrinza/prescribing-inform....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

