Honokiol suppresses formyl peptide-induced human neutrophil activation by blocking formyl peptide receptor 1
- PMID: 28751674
- PMCID: PMC5532207
- DOI: 10.1038/s41598-017-07131-w
Honokiol suppresses formyl peptide-induced human neutrophil activation by blocking formyl peptide receptor 1
Abstract
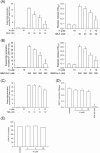
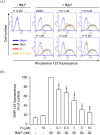
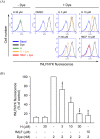
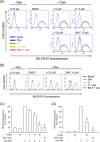
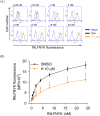
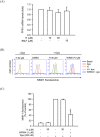
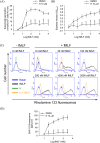
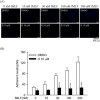
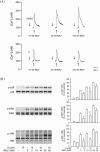
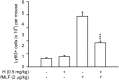
Formyl peptide receptor 1 (FPR1) mediates bacterial and mitochondrial N-formyl peptides-induced neutrophil activation. Therefore, FPR1 is an important therapeutic target for drugs to treat septic or sterile inflammatory diseases. Honokiol, a major bioactive compound of Magnoliaceae plants, possesses several anti-inflammatory activities. Here, we show that honokiol exhibits an inhibitory effect on FPR1 binding in human neutrophils. Honokiol inhibited superoxide anion generation, reactive oxygen species formation, and elastase release in bacterial or mitochondrial N-formyl peptides (FPR1 agonists)-activated human neutrophils. Adhesion of FPR1-induced human neutrophils to cerebral endothelial cells was also reduced by honokiol. The receptor-binding results revealed that honokiol repressed FPR1-specific ligand N-formyl-Nle-Leu-Phe-Nle-Tyr-Lys-fluorescein binding to FPR1 in human neutrophils, neutrophil-like THP-1 cells, and hFPR1-transfected HEK293 cells. However, honokiol did not inhibit FPR2-specific ligand binding to FPR2 in human neutrophils. Furthermore, honokiol inhibited FPR1 agonist-induced calcium mobilization as well as phosphorylation of p38 MAPK, ERK, and JNK in human neutrophils. In conclusion, our data demonstrate that honokiol may have therapeutic potential for treating FPR1-mediated inflammatory diseases.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

