Aurora-B kinase pathway controls the lateral to end-on conversion of kinetochore-microtubule attachments in human cells
- PMID: 28751710
- PMCID: PMC5532248
- DOI: 10.1038/s41467-017-00209-z
Aurora-B kinase pathway controls the lateral to end-on conversion of kinetochore-microtubule attachments in human cells
Abstract
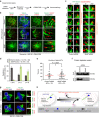
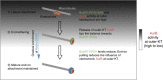
Human chromosomes are captured along microtubule walls (lateral attachment) and then tethered to microtubule-ends (end-on attachment) through a multi-step end-on conversion process. Upstream regulators that orchestrate this remarkable change in the plane of kinetochore-microtubule attachment in human cells are not known. By tracking kinetochore movements and using kinetochore markers specific to attachment status, we reveal a spatially defined role for Aurora-B kinase in retarding the end-on conversion process. To understand how Aurora-B activity is counteracted, we compare the roles of two outer-kinetochore bound phosphatases and find that BubR1-associated PP2A, unlike KNL1-associated PP1, plays a significant role in end-on conversion. Finally, we uncover a novel role for Aurora-B regulated Astrin-SKAP complex in ensuring the correct plane of kinetochore-microtubule attachment. Thus, we identify Aurora-B as a key upstream regulator of end-on conversion in human cells and establish a late role for Astrin-SKAP complex in the end-on conversion process.Human chromosomes are captured along microtubule walls and then tethered to microtubule-ends through a multi-step end-on conversion process. Here the authors show that Aurora-B regulates end-on conversion in human cells and establish a late role for Astrin-SKAP complex in the end-on conversion process.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
SKAP interacts with Aurora B to guide end-on capture of spindle microtubules via phase separation.J Mol Cell Biol. 2022 Jan 29;13(12):841-852. doi: 10.1093/jmcb/mjab058. J Mol Cell Biol. 2022. PMID: 34554241 Free PMC article.
-
Aurora B kinase controls the targeting of the Astrin-SKAP complex to bioriented kinetochores.J Cell Biol. 2010 Oct 18;191(2):269-80. doi: 10.1083/jcb.201006129. Epub 2010 Oct 11. J Cell Biol. 2010. PMID: 20937697 Free PMC article.
-
KNL1 facilitates phosphorylation of outer kinetochore proteins by promoting Aurora B kinase activity.J Cell Biol. 2013 Dec 23;203(6):957-69. doi: 10.1083/jcb.201306054. J Cell Biol. 2013. PMID: 24344188 Free PMC article.
-
Correcting aberrant kinetochore microtubule attachments: a hidden regulation of Aurora B on microtubules.Curr Opin Cell Biol. 2019 Jun;58:34-41. doi: 10.1016/j.ceb.2018.12.007. Epub 2019 Jan 23. Curr Opin Cell Biol. 2019. PMID: 30684807 Free PMC article. Review.
-
A Kinase-Phosphatase Network that Regulates Kinetochore-Microtubule Attachments and the SAC.Prog Mol Subcell Biol. 2017;56:457-484. doi: 10.1007/978-3-319-58592-5_19. Prog Mol Subcell Biol. 2017. PMID: 28840249 Review.
Cited by
-
RZZ-SPINDLY-DYNEIN: you got to keep 'em separated.Cell Cycle. 2020 Jul;19(14):1716-1726. doi: 10.1080/15384101.2020.1780382. Epub 2020 Jun 16. Cell Cycle. 2020. PMID: 32544383 Free PMC article. Review.
-
Lateral attachment of kinetochores to microtubules is enriched in prometaphase rosette and facilitates chromosome alignment and bi-orientation establishment.Sci Rep. 2018 Mar 1;8(1):3888. doi: 10.1038/s41598-018-22164-5. Sci Rep. 2018. PMID: 29497093 Free PMC article.
-
The right place at the right time: Aurora B kinase localization to centromeres and kinetochores.Essays Biochem. 2020 Sep 4;64(2):299-311. doi: 10.1042/EBC20190081. Essays Biochem. 2020. PMID: 32406506 Free PMC article. Review.
-
SKAP interacts with Aurora B to guide end-on capture of spindle microtubules via phase separation.J Mol Cell Biol. 2022 Jan 29;13(12):841-852. doi: 10.1093/jmcb/mjab058. J Mol Cell Biol. 2022. PMID: 34554241 Free PMC article.
-
Kinetochore- and chromosome-driven transition of microtubules into bundles promotes spindle assembly.Nat Commun. 2022 Nov 27;13(1):7307. doi: 10.1038/s41467-022-34957-4. Nat Commun. 2022. PMID: 36435852 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

