Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells
- PMID: 28751752
- PMCID: PMC5532219
- DOI: 10.1038/s41598-017-06803-x
Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells
Abstract
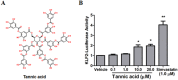
The transcription factor Kruppel-like factor 2 (KLF2) is a critical anti-inflammatory and anti-atherogenic molecule in vascular endothelium. Enhancing KLF2 expression and activity improves endothelial function and prevents atherosclerosis. However, the pharmacological and molecular regulators for KLF2 are scarce. Using high-throughput luciferase reporter assay to screen for KLF2 activators, we have identified tannic acid (TA), a polyphenolic compound, as a potent KLF2 activator that attenuates endothelial inflammation. Mechanistic studies suggested that TA induced KLF2 expression in part through the ERK5/MEF2 pathway. Functionally, TA markedly decreased monocyte adhesion to ECs by reducing expression of adhesion molecule VCAM1. Using lung ECs isolated from Klf2 +/+ and Klf2 +/- mice, we showed that the anti-inflammatory effect of TA is dependent on KLF2. Collectively, our results demonstrate that TA is a potent KLF2 activator and TA attenuated endothelial inflammation through upregulation of KLF2. Our findings provide a novel mechanism for the well-established beneficial cardiovascular effects of TA and suggest that KLF2 could be a novel therapeutic target for atherosclerotic vascular disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Suberanilohydroxamic Acid as a Pharmacological Kruppel-Like Factor 2 Activator That Represses Vascular Inflammation and Atherosclerosis.J Am Heart Assoc. 2017 Nov 30;6(12):e007134. doi: 10.1161/JAHA.117.007134. J Am Heart Assoc. 2017. PMID: 29191808 Free PMC article.
-
Soy-Leaf Extract Exerts Atheroprotective Effects via Modulation of Krüppel-Like Factor 2 and Adhesion Molecules.Int J Mol Sci. 2017 Feb 10;18(2):373. doi: 10.3390/ijms18020373. Int J Mol Sci. 2017. PMID: 28208647 Free PMC article.
-
Angiopoietin-1 induces Kruppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2.J Biol Chem. 2009 Feb 27;284(9):5592-601. doi: 10.1074/jbc.M806928200. Epub 2008 Dec 23. J Biol Chem. 2009. PMID: 19106103
-
Endothelial Krüppel-like factor 2/4: Regulation and function in cardiovascular diseases.Cell Signal. 2025 Jun;130:111699. doi: 10.1016/j.cellsig.2025.111699. Epub 2025 Feb 27. Cell Signal. 2025. PMID: 40023301 Review.
-
Transcription Factor KLF2 and Its Role in the Regulation of Inflammatory Processes.Biochemistry (Mosc). 2020 Jan;85(1):54-67. doi: 10.1134/S0006297920010058. Biochemistry (Mosc). 2020. PMID: 32079517 Review.
Cited by
-
Possible Beneficial Effects of Hydrolyzable Tannins Deriving from Castanea sativa L. in Internal Medicine.Nutrients. 2023 Dec 22;16(1):45. doi: 10.3390/nu16010045. Nutrients. 2023. PMID: 38201875 Free PMC article. Review.
-
The Role of KLF2 in the Regulation of Atherosclerosis Development and Potential Use of KLF2-Targeted Therapy.Biomedicines. 2022 Jan 24;10(2):254. doi: 10.3390/biomedicines10020254. Biomedicines. 2022. PMID: 35203463 Free PMC article. Review.
-
Krϋppel-like factors (KLFs) in renal physiology and disease.EBioMedicine. 2019 Feb;40:743-750. doi: 10.1016/j.ebiom.2019.01.021. Epub 2019 Jan 17. EBioMedicine. 2019. PMID: 30662001 Free PMC article. Review.
-
A simple protocol for isolating mouse lung endothelial cells.Sci Rep. 2019 Feb 6;9(1):1458. doi: 10.1038/s41598-018-37130-4. Sci Rep. 2019. PMID: 30728372 Free PMC article.
-
Neutrophil-fibroblast crosstalk drives immunofibrosis in Crohn's disease through IFNα pathway.Front Immunol. 2024 Sep 13;15:1447608. doi: 10.3389/fimmu.2024.1447608. eCollection 2024. Front Immunol. 2024. PMID: 39346917 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

