CTLA4-CD28 chimera gene modification of T cells enhances the therapeutic efficacy of donor lymphocyte infusion for hematological malignancy
- PMID: 28751785
- PMCID: PMC5565951
- DOI: 10.1038/emm.2017.104
CTLA4-CD28 chimera gene modification of T cells enhances the therapeutic efficacy of donor lymphocyte infusion for hematological malignancy
Abstract
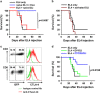
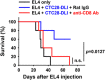
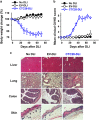
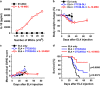
Donor lymphocyte infusion (DLI) followed by hematopoietic stem cell transplantation has served as an effective prevention/treatment modality against the relapse of some hematologic tumors, such as chronic myeloid leukemia (CML). However, the therapeutic efficacies of DLI for other types of leukemia, including acute lymphocytic leukemia (ALL), have been limited thus far. Therefore, we examined whether increasing the reactivity of donor T cells by gene modification could enhance the therapeutic efficacy of DLI in a murine model of ALL. When a CTLA4-CD28 chimera gene (CTC28) in which the intracellular signaling domain of CTLA4 was replaced with the CD28 signaling domain was introduced into CD4 and CD8 T cells in DLI, the graft-versus-tumor (GVT) effect was significantly increased. This effect was correlated with an increased expansion of donor CD8 T cells in vivo, and the depletion of CD8 T cells abolished this effect. The CD8 T cell expansion and the enhanced GVT effect were dependent on the transduction of both CD4 and CD8 T cells with CTC28, which emphasizes the role of dual modification in this therapeutic effect. The CTC28-transduced T cells that expanded in vivo also exhibited enhanced functionality. Although the potentiation of the GVT effect mediated by the CTC28 gene modification of T cells was accompanied by an increase of graft-versus-host disease (GVHD), the GVHD was not lethal and was mitigated by treatment with IL-10 gene-modified third-party mesenchymal stem cells. Thus, the combined genetic modification of CD4 and CD8 donor T cells with CTC28 could be a promising strategy for enhancing the therapeutic efficacy of DLI.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
During acute graft versus host disease CD28 deletion in donor CD8+ , but not CD4+ , T cells maintain antileukemia responses in mice.Eur J Immunol. 2018 Dec;48(12):2055-2067. doi: 10.1002/eji.201847669. Epub 2018 Nov 14. Eur J Immunol. 2018. PMID: 30320878
-
Graft-vs.-host and graft-vs.-leukemia reactions after delayed infusions of donor T-subsets.Biol Blood Marrow Transplant. 1999;5(3):123-32. doi: 10.1053/bbmt.1999.v5.pm10392958. Biol Blood Marrow Transplant. 1999. PMID: 10392958
-
Host T cells resist graft-versus-host disease mediated by donor leukocyte infusions.J Immunol. 2000 Nov 1;165(9):4901-9. doi: 10.4049/jimmunol.165.9.4901. J Immunol. 2000. PMID: 11046015
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Donor lymphocyte infusion: the use of alloreactive and tumor-reactive lymphocytes for immunotherapy of malignant and nonmalignant diseases in conjunction with allogeneic stem cell transplantation.J Hematother Stem Cell Res. 2002 Apr;11(2):265-76. doi: 10.1089/152581602753658457. J Hematother Stem Cell Res. 2002. PMID: 11983098 Review.
Cited by
-
Overview of Cellular Immunotherapies within Transfusion Medicine for the Treatment of Malignant Diseases.Int J Mol Sci. 2021 May 12;22(10):5120. doi: 10.3390/ijms22105120. Int J Mol Sci. 2021. PMID: 34066067 Free PMC article. Review.
-
Modular pooled discovery of synthetic knockin sequences to program durable cell therapies.Cell. 2023 Sep 14;186(19):4216-4234.e33. doi: 10.1016/j.cell.2023.08.013. Cell. 2023. PMID: 37714135 Free PMC article.
-
Gene modification strategies for next-generation CAR T cells against solid cancers.J Hematol Oncol. 2020 May 18;13(1):54. doi: 10.1186/s13045-020-00890-6. J Hematol Oncol. 2020. PMID: 32423475 Free PMC article. Review.
-
Chimeric non-antigen receptors in T cell-based cancer therapy.J Immunother Cancer. 2021 Aug;9(8):e002628. doi: 10.1136/jitc-2021-002628. J Immunother Cancer. 2021. PMID: 34344725 Free PMC article. Review.
-
Chimeric CTLA4-CD28-CD3z T Cells Potentiate Antitumor Activity Against CD80/CD86-Positive B Cell Malignancies.Front Immunol. 2021 Apr 2;12:642528. doi: 10.3389/fimmu.2021.642528. eCollection 2021. Front Immunol. 2021. PMID: 33868277 Free PMC article.
References
-
- Jenq RR, van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer 2010; 10: 213–221. - PubMed
-
- Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 1997; 90: 3204–3213. - PubMed
-
- Spitzer TR, McAfee S, Sackstein R, Colby C, Toh HC, Multani P et al. Intentional induction of mixed chimerism and achievement of antitumor responses after nonmyeloablative conditioning therapy and HLA-matched donor bone marrow transplantation for refractory hematologic malignancies. Biol Blood Marrow Transplant 2000; 6(3A): 309–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

