Overexpression of SbSI-1, A Nuclear Protein from Salicornia brachiata Confers Drought and Salt Stress Tolerance and Maintains Photosynthetic Efficiency in Transgenic Tobacco
- PMID: 28751902
- PMCID: PMC5508026
- DOI: 10.3389/fpls.2017.01215
Overexpression of SbSI-1, A Nuclear Protein from Salicornia brachiata Confers Drought and Salt Stress Tolerance and Maintains Photosynthetic Efficiency in Transgenic Tobacco
Abstract
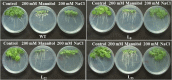
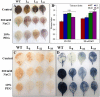
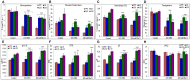
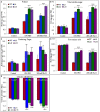
A novel Salicornia brachiata Salt Inducible (SbSI-1) gene was isolated and overexpressed in tobacco for in planta functional validation subjected to drought and salt stress. SbSI-1 is a nuclear protein. The transgenic tobacco overexpressing SbSI-1 gene exhibited better seed germination, growth performances, pigment contents, cell viability, starch accumulation, and tolerance index under drought and salt stress. Overexpression of SbSI-1 gene alleviated the build-up of reactive oxygen species (ROS) and curtailed the ROS-induced oxidative damages thus improved the physiological health of transgenic tobacco under stressed conditions. The higher activities of antioxidant enzymes, lower accumulation of ROS, higher membrane stability, relative water content, and polyphenol contents indicated the better survival of the transgenic tobacco than wild-type (WT) tobacco under stressed conditions. Transgenic tobacco had a higher net photosynthetic rate, PSII operating efficiency, and performance index under drought and salt stress. Higher accumulation of compatible solutes and K+/Na+ ratio in transgenic tobacco than WT showed the better osmotic and redox homeostasis under stressed conditions. The up-regulation of genes encoding antioxidant enzymes (NtSOD, NtAPX, and NtCAT) and transcription factors (NtDREB2 and NtAP2) in transgenic tobacco under stressed conditions showed the role of SbSI-1 in ROS alleviation and involvement of this gene in abiotic stress tolerance. Multivariate data analysis exhibited statistical distinction among growth responses, physiological health, osmotic adjustment, and photosynthetic responses of WT and transgenic tobacco under stressed conditions. The overexpression of SbSI-1 gene curtailed the ROS-induced oxidative damages and maintained the osmotic homeostasis under stress conditions thus improved physiological health and photosynthetic efficiencies of the transgenic tobacco overexpressing SbSI-1 gene.
Keywords: abiotic stress; and transgenic; drought; halophyte; oxidative damage; salt.
Figures







Similar articles
-
A novel gene SbSI-2 encoding nuclear protein from a halophyte confers abiotic stress tolerance in E. coli and tobacco.PLoS One. 2014 Jul 7;9(7):e101926. doi: 10.1371/journal.pone.0101926. eCollection 2014. PLoS One. 2014. PMID: 24999628 Free PMC article.
-
The novel galactosyl transferase-like (SbGalT) gene from Salicornia brachiata maintains photosynthesis and enhances abiotic stress tolerance in transgenic tobacco.Gene. 2021 Jun 20;786:145597. doi: 10.1016/j.gene.2021.145597. Epub 2021 Mar 23. Gene. 2021. PMID: 33766708
-
A SNARE-Like Superfamily Protein SbSLSP from the Halophyte Salicornia brachiata Confers Salt and Drought Tolerance by Maintaining Membrane Stability, K(+)/Na(+) Ratio, and Antioxidant Machinery.Front Plant Sci. 2016 Jun 2;7:737. doi: 10.3389/fpls.2016.00737. eCollection 2016. Front Plant Sci. 2016. PMID: 27313584 Free PMC article.
-
Expansins in Salt and Drought Stress Adaptation: From Genome-Wide Identification to Functional Characterisation in Crops.Plants (Basel). 2025 Apr 28;14(9):1327. doi: 10.3390/plants14091327. Plants (Basel). 2025. PMID: 40364355 Free PMC article. Review.
-
Responses of Membranes and the Photosynthetic Apparatus to Salt Stress in Cyanobacteria.Front Plant Sci. 2020 Jun 5;11:713. doi: 10.3389/fpls.2020.00713. eCollection 2020. Front Plant Sci. 2020. PMID: 32582247 Free PMC article. Review.
Cited by
-
Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants.Int J Mol Sci. 2021 Oct 3;22(19):10733. doi: 10.3390/ijms221910733. Int J Mol Sci. 2021. PMID: 34639074 Free PMC article. Review.
-
Assessing the potential of exogenous caffeic acid application in boosting wheat (Triticum aestivum L.) crop productivity under salt stress.PLoS One. 2021 Nov 2;16(11):e0259222. doi: 10.1371/journal.pone.0259222. eCollection 2021. PLoS One. 2021. PMID: 34727104 Free PMC article.
-
Analysis of the Role of the Drought-Induced Gene DRI15 and Salinity-Induced Gene SI1 in Alternanthera philoxeroides Plasticity Using a Virus-Based Gene Silencing Tool.Front Plant Sci. 2017 Sep 12;8:1579. doi: 10.3389/fpls.2017.01579. eCollection 2017. Front Plant Sci. 2017. PMID: 28955366 Free PMC article.
-
Impact of homologous overexpression of PR10a gene on improving salt stress tolerance in transgenic Solanum tuberosum.J Genet Eng Biotechnol. 2024 Dec;22(4):100437. doi: 10.1016/j.jgeb.2024.100437. Epub 2024 Nov 13. J Genet Eng Biotechnol. 2024. PMID: 39674650 Free PMC article.
-
Introgression of UfCyt c6, a thylakoid lumen protein from a green seaweed Ulva fasciata Delile enhanced photosynthesis and growth in tobacco.Mol Biol Rep. 2018 Dec;45(6):1745-1758. doi: 10.1007/s11033-018-4318-1. Epub 2018 Aug 29. Mol Biol Rep. 2018. PMID: 30159639
References
-
- Able A. J., Guest D. I., Sutherland M. W. (1998). Use of a new Tetrazolium-based assay to study the production of superoxide radicals by Tobacco cell cultures challenged with avirulent zoospores of phytophthora parasitica var nicotianae. Plant Physiol. 117, 491–499. 10.1104/pp.117.2.491 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

