Chk1 Promotes DNA Damage Response Bypass following Oxidative Stress in a Model of Hydrogen Peroxide-Associated Ulcerative Colitis through JNK Inactivation and Chromatin Binding
- PMID: 28751935
- PMCID: PMC5478872
- DOI: 10.1155/2017/9303158
Chk1 Promotes DNA Damage Response Bypass following Oxidative Stress in a Model of Hydrogen Peroxide-Associated Ulcerative Colitis through JNK Inactivation and Chromatin Binding
Abstract
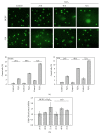
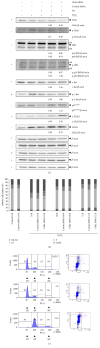
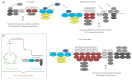
Dysregulation of c-Jun N-terminal kinase (JNK) activation promoted DNA damage response bypass and tumorigenesis in our model of hydrogen peroxide-associated ulcerative colitis (UC) and in patients with quiescent UC (QUC), UC-related dysplasia, and UC-related carcinoma (UC-CRC), thereby adapting to oxidative stress. In the UC model, we have observed features of oncogenic transformation: increased proliferation, undetected DNA damage, and apoptosis resistance. Here, we show that Chk1 was downregulated but activated in the acute and quiescent chronic phases. In both phases, Chk1 was linked to DNA damage response bypass by suppressing JNK activation following oxidative stress, promoting cell cycle progression despite DNA damage. Simultaneously, activated Chk1 was bound to chromatin. This triggered histone acetylation and the binding of histone acetyltransferases and transcription factors to chromatin. Thus, chromatin-immobilized activated Chk1 executed a dual function by suppressing DNA damage response and simultaneously inducing chromatin modulation. This caused undetected DNA damage and increased cellular proliferation through failure to transmit the appropriate DNA damage signal. Findings in vitro were corroborated by chromatin accumulation of activated Chk1, Ac-H3, Ac-H4, and c-Jun in active UC (AUC) in vivo. Targeting chromatin-bound Chk1, GCN5, PCAF, and p300/CBP could be a novel therapeutic strategy to prevent UC-related tumor progression.
Figures







Similar articles
-
Inactivation of JNK2 as carcinogenic factor in colitis-associated and sporadic colorectal carcinogenesis.Carcinogenesis. 2017 May 1;38(5):559-569. doi: 10.1093/carcin/bgx032. Carcinogenesis. 2017. PMID: 28383667
-
Repeated H2 O2 exposure drives cell cycle progression in an in vitro model of ulcerative colitis.J Cell Mol Med. 2013 Dec;17(12):1619-31. doi: 10.1111/jcmm.12150. Epub 2013 Oct 9. J Cell Mol Med. 2013. PMID: 24118792 Free PMC article.
-
Non-apoptotic function of caspases in a cellular model of hydrogen peroxide-associated colitis.J Cell Mol Med. 2013 Jul;17(7):901-13. doi: 10.1111/jcmm.12079. Epub 2013 Jun 7. J Cell Mol Med. 2013. PMID: 23742011 Free PMC article.
-
The fork and the kinase: a DNA replication tale from a CHK1 perspective.Mutat Res Rev Mutat Res. 2015 Jan-Mar;763:168-80. doi: 10.1016/j.mrrev.2014.10.003. Epub 2014 Oct 22. Mutat Res Rev Mutat Res. 2015. PMID: 25795119 Free PMC article. Review.
-
CHK1 and replicative stress in T-cell leukemia: Can an irreverent tumor suppressor end up playing the oncogene?Adv Biol Regul. 2016 Jan;60:115-121. doi: 10.1016/j.jbior.2015.10.007. Epub 2015 Oct 24. Adv Biol Regul. 2016. PMID: 26527132 Review.
Cited by
-
Ginkgo Biloba L. Extract Reduces H2O2-Induced Bone Marrow Mesenchymal Stem Cells Cytotoxicity by Regulating Mitogen-Activated Protein Kinase (MAPK) Signaling Pathways and Oxidative Stress.Med Sci Monit. 2018 May 14;24:3159-3167. doi: 10.12659/MSM.910718. Med Sci Monit. 2018. PMID: 29758019 Free PMC article.
-
Keratinocytes Migration Promotion, Proliferation Induction, and Free Radical Injury Prevention by 3-Hydroxytirosol.Int J Mol Sci. 2021 Feb 28;22(5):2438. doi: 10.3390/ijms22052438. Int J Mol Sci. 2021. PMID: 33670966 Free PMC article.
-
Inhibiting Ferroptosis: A Novel Approach for Ulcerative Colitis Therapeutics.Oxid Med Cell Longev. 2022 Mar 26;2022:9678625. doi: 10.1155/2022/9678625. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35378823 Free PMC article. Review.
-
Recent Approaches to Determine Static and Dynamic Redox State-Related Parameters.Antioxidants (Basel). 2022 Apr 28;11(5):864. doi: 10.3390/antiox11050864. Antioxidants (Basel). 2022. PMID: 35624728 Free PMC article. Review.
References
-
- Arai N., Mitomi H., Ohtani Y., Igarashi M., Kakita A., Okayasu I. Enhanced epithelial cell turnover associated with p53 accumulation and high p21WAF1/CIP1 expression in ulcerative colitis. Modern Pathology. 1999;12(6):604–611. - PubMed
-
- MacFie T. S., Poulsom R., Parker A., et al. DUOX2 and DUOXA2 form the predominant enzyme system capable of producing the reactive oxygen species H2O2 in active ulcerative colitis and are modulated by 5-aminosalicylic acid. Inflammatory Bowel Diseases. 2014;20(3):514–524. doi: 10.1097/01.MIB.0000442012.45038.0e. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

