Automated Image Analysis of HER2 Fluorescence In Situ Hybridization to Refine Definitions of Genetic Heterogeneity in Breast Cancer Tissue
- PMID: 28752092
- PMCID: PMC5511668
- DOI: 10.1155/2017/2321916
Automated Image Analysis of HER2 Fluorescence In Situ Hybridization to Refine Definitions of Genetic Heterogeneity in Breast Cancer Tissue
Abstract
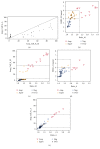
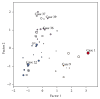
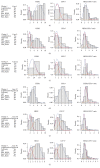
Human epidermal growth factor receptor 2 gene- (HER2-) targeted therapy for breast cancer relies primarily on HER2 overexpression established by immunohistochemistry (IHC) with borderline cases being further tested for amplification by fluorescence in situ hybridization (FISH). Manual interpretation of HER2 FISH is based on a limited number of cells and rather complex definitions of equivocal, polysomic, and genetically heterogeneous (GH) cases. Image analysis (IA) can extract high-capacity data and potentially improve HER2 testing in borderline cases. We investigated statistically derived indicators of HER2 heterogeneity in HER2 FISH data obtained by automated IA of 50 IHC borderline (2+) cases of invasive ductal breast carcinoma. Overall, IA significantly underestimated the conventional HER2, CEP17 counts, and HER2/CEP17 ratio; however, it collected more amplified cells in some cases below the lower limit of GH definition by manual procedure. Indicators for amplification, polysomy, and bimodality were extracted by factor analysis and allowed clustering of the tumors into amplified, nonamplified, and equivocal/polysomy categories. The bimodality indicator provided independent cell diversity characteristics for all clusters. Tumors classified as bimodal only partially coincided with the conventional GH heterogeneity category. We conclude that automated high-capacity nonselective tumor cell assay can generate evidence-based HER2 intratumor heterogeneity indicators to refine GH definitions.
Figures

References
-
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. - PubMed
-
- Wolff A. C., Hammond M. E., Hicks D. G. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Journal Clinical Oncology. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. - DOI - PubMed
-
- Hicks D. G., Kulkarni S. Trastuzumab as adjuvant therapy for early breast cancer: the importance of accurate human epidermal growth factor receptor 2 testing. Archives of Pathology & Laboratory Medicine. 2008;132(6):1008–1015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

