Safety Profile of Eslicarbazepine Acetate as Add-On Therapy in Adults with Refractory Focal-Onset Seizures: From Clinical Studies to 6 Years of Post-Marketing Experience
- PMID: 28752473
- PMCID: PMC5688182
- DOI: 10.1007/s40264-017-0576-4
Safety Profile of Eslicarbazepine Acetate as Add-On Therapy in Adults with Refractory Focal-Onset Seizures: From Clinical Studies to 6 Years of Post-Marketing Experience
Abstract
Introduction: Eslicarbazepine acetate was first approved in the European Union in 2009 as adjunctive therapy in adults with partial-onset seizures with or without secondary generalization.
Objective: The objective of this study was to review the safety profile of eslicarbazepine acetate analyzing the data from several clinical studies to 6 years of post-marketing surveillance.
Methods: We used a post-hoc pooled safety analysis of four phase III, double-blind, randomized, placebo-controlled studies (BIA-2093-301, -302, -303, -304) of eslicarbazepine acetate as add-on therapy in adults. Safety data of eslicarbazepine acetate in special populations of patients aged ≥65 years with partial-onset seizures (BIA-2093-401) and subjects with moderate hepatic impairment (BIA-2093-111) and renal impairment (BIA-2093-112) are also considered. The incidences of treatment-emergent adverse events, treatment-emergent adverse events leading to discontinuation, and serious adverse events were analyzed. The global safety database of eslicarbazepine acetate was analyzed for all cases from post-marketing surveillance from 1 October, 2009 to 21 October, 2015.
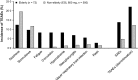
Results: From a pooled analysis of four phase III studies, it was concluded that the incidence of treatment-emergent adverse events, treatment-emergent adverse events leading to discontinuation, and adverse drug reactions were dose dependent. Dizziness, somnolence, headache, and nausea were the most common treatment-emergent adverse events (≥10% of patients) and the majority were of mild-to-moderate intensity. No dose-dependent trend was observed for serious adverse events and individual serious adverse events were reported in less than 1% of patients. Hyponatremia was classified as a possibly related treatment-emergent adverse event in phase III studies (1.2%); however, after 6 years of post-marketing surveillance it represents the most frequently (10.2%) reported adverse drug reaction, with more than half of these cases occurring with eslicarbazepine acetate at daily doses of 1200 mg. Other adverse drug reactions reported in post-marketing surveillance are seizure (5.8%), dizziness (4.1%), rash (2.6%), and fatigue (2.1%). The safety profile of eslicarbazepine acetate in renal and hepatic impairment subjects (phase I studies) and in elderly patients (phase III study) did not raise any specific concern.
Conclusion: After 6 years of post-marketing surveillance, eslicarbazepine acetate maintains a similar safety profile to that observed in pivotal clinical studies.
Conflict of interest statement
Funding
Bial–Portela & Ca, S.A., Coronado, Portugal and Sunovion Pharmaceuticals Inc., Marlborough, MA, USA funded the phase III clinical trials reported in this manuscript. The sponsors were involved in the study design, in the collection, analysis, and interpretation of data, writing of the report, and the decision to submit the article for publication.
Conflict of interest
Helena Gama, Mariana Vieira, Raquel Costa, Joana Graça, Luís M. Magalhães, and Patrício Soares-da-Silva are employees of Bial–Portela & Cª, S.A.
Figures
References
-
- European Medicines Agency. Zebinix (eslicarbazepine acetate): EU summary of product characteristics. 2017. Available from: http://www.ema.europa.eu/ema/. Accessed 15 July 2017.
-
- Sunovion Pharmaceuticals Inc. Aptiom® (eslicarbazepine acetate) tablets, for oral use: US prescribing information. 2015. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022416s001lbl.pdf. Accessed 15 July 2017.
-
- Elger C, Halasz P, Maia J, Almeida L, Soares-da-Silva P. Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures: a randomized, double-blind, placebo-controlled, parallel-group phase III study. Epilepsia. 2009;50(3):454–463. doi: 10.1111/j.1528-1167.2008.01946.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

