Oral versus intravenous fluoropyrimidines for colorectal cancer
- PMID: 28752564
- PMCID: PMC6483122
- DOI: 10.1002/14651858.CD008398.pub2
Oral versus intravenous fluoropyrimidines for colorectal cancer
Abstract
Background: Patients prefer oral to intravenous (IV) palliative chemotherapy, provided that oral therapy is not less effective. We compared the efficacy and safety of oral and IV fluoropyrimidines for treatment of colorectal cancer (CRC).
Objectives: To compare the effects of oral and IV fluoropyrimidine chemotherapy in patients treated with curative or palliative intent for CRC.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5), along with OVID MEDLINE, OVID Embase, and Web of Science databases, in June 2016. We also searched five clinical trials registers, several conference proceedings, and reference lists from study reports and systematic reviews. We contacted pharmaceutical companies to identify additional studies.
Selection criteria: We included randomised controlled trials (RCTs) comparing oral and IV fluoropyrimidine chemotherapy in patients treated with curative or palliative intent for CRC.
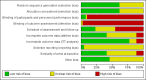
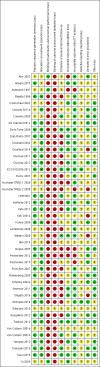
Data collection and analysis: Three review authors extracted data and assessed risk of bias independently. We assessed the seven domains in the Cochrane 'Risk of bias' tool and three additional domains: schedules of outcome assessment and/or follow-up; use of intention-to-treat analysis; and baseline comparability of treatment arms.
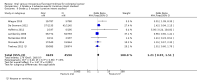
Main results: We included nine RCTs (total of 10,918 participants) that examined treatment with curative intent for CRC with neoadjuvant and/or adjuvant chemotherapy. We included 35 RCTs (total of 12,592 participants) that examined treatment with palliative intent for inoperable advanced or metastatic CRC with chemotherapy (31 first-line studies, two second-line studies, and two studies of first- or second-line chemotherapy). All studies included male and female participants, and no studies included participants younger than 18 years of age. Patients treated with curative intent for CRC with neoadjuvant and/or adjuvant chemotherapy • Disease-free survival (DFS): DFS did not differ between participants treated with oral versus IV fluoropyrimidines (hazard ratio (HR) 0.93, 95% confidence interval (CI) 0.87 to 1.00; seven studies, 8903 participants; moderate-quality evidence).• Overall survival (OS): OS did not differ between participants treated with oral versus IV fluoropyrimidines (HR 0.92, 95% CI 0.84 to 1.00; seven studies, 8902 participants analysed; high-quality evidence).• Grade ≥ 3 adverse events (AEs): Participants treated with oral fluoropyrimidines experienced less grade ≥ 3 neutropenia/granulocytopenia (odds ratio (OR) 0.14, 95% CI 0.11 to 0.16; seven studies, 8087 participants; moderate-quality evidence), stomatitis (OR 0.21, 95% CI 0.14 to 0.30; five studies, 4212 participants; low-quality evidence), and any grade ≥ 3 AEs (OR 0.82, 95% CI 0.74 to 0.90; five studies, 7741 participants; low-quality evidence). There was more grade ≥ 3 hand foot syndrome (OR 4.59, 95% CI 2.97 to 7.10; five studies, 5731 participants; low-quality evidence) in patients treated with oral fluoropyrimidines. There were no differences between participants treated with oral versus IV fluoropyrimidines in occurrence of grade ≥ 3 diarrhoea (OR 1.12, 95% CI 0.99 to 1.25; nine studies, 9551 participants; very low-quality evidence), febrile neutropenia (OR 0.59, 95% CI 0.18 to 1.90; four studies, 2925 participants; low-quality evidence), vomiting (OR 1.05, 95% CI 0.83 to 1.34; eight studies, 9385 participants; low-quality evidence), nausea (OR 1.21, 95% CI 0.97 to 1.51; seven studies, 9233 participants; low-quality evidence), mucositis (OR 0.64, 95% CI 0.25 to 1.62; four studies, 2233 participants; very low-quality evidence), and hyperbilirubinaemia (OR 1.67, 95% CI 0.52 to 5.38; three studies, 2757 participants; very low-quality evidence). Patients treated with palliative intent for inoperable advanced or metastatic CRC with chemotherapy • Progression-free survival (PFS): Overall, PFS was inferior in participants treated with oral versus IV fluoropyrimidines (HR 1.06, 95% CI 1.02 to 1.11; 23 studies, 9927 participants; moderate-quality evidence). Whilst PFS was worse in participants treated with oral compared with IV fluoropyrimidines when UFT/Ftorafur or eniluracil with oral 5-fluorouracil (5-FU) was used, PFS did not differ between individuals treated with oral versus IV fluoropyrimidines when capecitabine, doxifluridine, or S-1 was used.• OS: Overall, OS did not differ between participants treated with oral versus IV fluoropyrimidines (HR 1.02, 95% CI 0.99 to 1.05; 29 studies, 12,079 participants; high-quality evidence). OS was inferior in participants treated with oral versus IV fluoropyrimidines when eniluracil with oral 5-fluorouracil (5-FU) was used.• Time to progression (TTP): TTP was inferior in participants treated with oral versus IV fluoropyrimidines (HR 1.07, 95% CI 1.01 to 1.14; six studies, 1970 participants; moderate-quality evidence).• Objective response rate (ORR): ORR did not differ between participants treated with oral versus IV fluoropyrimidines (OR 0.98, 95% CI 0.90 to 1.06; 32 studies, 11,115 participants; moderate-quality evidence).• Grade ≥ 3 AEs: Participants treated with oral fluoropyrimidines experienced less grade ≥ 3 neutropenia/granulocytopenia (OR 0.17, 95% CI 0.15 to 0.18; 29 studies, 11,794 participants; low-quality evidence), febrile neutropenia (OR 0.27, 95% CI 0.21 to 0.36; 19 studies, 9407 participants; moderate-quality evidence), stomatitis (OR 0.26, 95% CI 0.20 to 0.33; 21 studies, 8718 participants; low-quality evidence), mucositis (OR 0.17, 95% CI 0.12 to 0.24; 12 studies, 4962 participants; low-quality evidence), and any grade ≥ 3 AEs (OR 0.83, 95% CI 0.74 to 0.94; 14 studies, 5436 participants; low-quality evidence). There was more grade ≥ 3 diarrhoea (OR 1.66, 95% CI 1.50 to 1.84; 30 studies, 11,997 participants; low-quality evidence) and hand foot syndrome (OR 3.92, 95% CI 2.84 to 5.43; 18 studies, 6481 participants; moderate-quality evidence) in the oral fluoropyrimidine arm. There were no differences between oral and IV fluoropyrimidine arms in terms of grade ≥ 3 vomiting (OR 1.18, 95% CI 1.00 to 1.40; 23 studies, 9528 participants; low-quality evidence), nausea (OR 1.16, 95% CI 0.99 to 1.36; 25 studies, 9796 participants; low-quality evidence), and hyperbilirubinaemia (OR 1.62, 95% CI 0.99 to 2.64; nine studies, 2699 participants; low-quality evidence).
Authors' conclusions: Results of this review should provide confidence that treatment for CRC with most of the oral fluoropyrimidines commonly used in current clinical practice is similarly efficacious to treatment with IV fluoropyrimidines. Treatment with eniluracil with oral 5-FU was associated with inferior PFS and OS among participants treated with palliative intent for CRC, and eniluracil is no longer being developed. Oral and IV fluoropyrimidines have different patterns of side effects; future research may focus on determining the basis for these differences.
Conflict of interest statement
FC has been provided with honorarium from Roche to speak at a non‐promotional educational meeting. DL has received an educational grant from Roche. TP is on advisory boards for Roche and Amgen. NT is on advisory boards for Roche, Amgen and Bayer.
Figures

























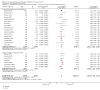






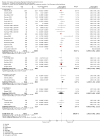


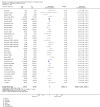
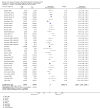

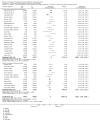
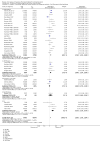
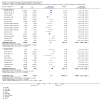




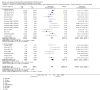







Update of
References
References to studies included in this review
Ahn 2003 {published data only (unpublished sought but not used)}
-
- Ahn J‐H, Kim T‐W, Lee J‐H, Min Y‐J, Kim J‐G, Kim JC, et al. Oral doxifluridine plus leucovorin in metastatic colorectal cancer: randomized phase II trial with intravenous 5‐fluorouracil plus leucovorin. American Journal of Clinical Oncology 2003;26(1):98‐102. - PubMed
Allegra 2015 {published and unpublished data}
-
- Allegra CJ, Yothers G, O'Connell MJ, Roh MS, Beart RW, Petrelli NJ, et al. Neoadjuvant therapy for rectal cancer: mature results from NSABP protocol R‐04. Journal of Clinical Oncology 2014;32(Suppl 3):abstr 390.
-
- Allegra CJ, Yothers G, O'Connell MJ, Roh MS, Lopa SH, Petrelli NJ, et al. Final results from NSABP protocol R‐04: neoadjuvant chemoradiation (RT) comparing continuous infusion (CIV) 5‐FU with capecitabine (Cape) with or without oxaliplatin (Ox) in patients with stage II and III rectal cancer. Journal of Clinical Oncology 2014;32(5s):abstr 3603.
-
- O'Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R‐04. Journal of Clinical Oncology 2014;32(18):1927‐34. - PMC - PubMed
-
- Roh MS, Yothers GA, O'Connell MJ, Beart RW, Pitot HC, Shields AF, et al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R‐04. Journal of Clinical Oncology 2011;29(Suppl):abstr 3503.
Andersen 1987 {published data only (unpublished sought but not used)}
-
- Andersen E, Pedersen H. Oral ftorafur versus intravenous 5‐fluorouracil. A comparative study in patients with colorectal cancer. Acta Oncologica 1987;26(6):433‐6. - PubMed
Bajetta 1996 {published data only (unpublished sought but not used)}
-
- Bajetta E, DiBartolomeo M, Somma L, Moreschi M, Comella G, Turci D, et al. Randomized phase II noncomparative trial of oral and intravenous doxifluridine plus levo‐leucovorin in untreated patients with advanced colorectal carcinoma. Cancer 1996;78(10):2087‐93. - PubMed
Carmichael 2002 {published data only (unpublished sought but not used)}
-
- Carmichael J, Popiela T, Radstone D, Falk S, Borner M, Oza A, et al. Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. Journal of Clinical Oncology 2002;20(17):3617‐27. - PubMed
Cassidy 2011a {published data only (unpublished sought but not used)}
-
- Arkenau HT, Arnold D, Cassidy J, Diaz‐Rubio E, Douillard JY, Hochster H, et al. Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. Journal of Clinical Oncology 2008;26(36):5910‐7. - PubMed
-
- Cassidy J, Clarke S, Diaz‐Rubio E, Scheithauer W, Figer A, Wong R, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first‐line therapy for metastatic colorectal cancer. Journal of Clinical Oncology 2008;26(12):2006‐12. - PubMed
Comella 2009 {published data only (unpublished sought but not used)}
-
- Comella P, Massidda B, Filippelli G, Farris A, Natale D, Barberis G, et al. Randomised trial comparing biweekly oxaliplatin plus oral capecitabine versus oxaliplatin plus i.v. bolus fluorouracil/leucovorin in metastatic colorectal cancer patients: results of the Southern Italy Cooperative Oncology study 0401. Journal of Cancer Research and Clinical Oncology 2009;135(2):217‐26. - PMC - PubMed
-
- Comella P, Massidda B, Filippelli G, Farris A, Natale D, Barberis G, et al. Randomized comparison of capecitabine versus fluorouracil/leucovorin in combination with oxaliplatin in metastatic colorectal cancer patients: Southern Italy Cooperative Oncology Group trial 0401. ASCO 2008 Gastrointestinal Cancers Symposium. 2008:abstr 344.
-
- Comella P, Massidda B, Filippelli G, Farris A, Natale D, Maiorino L, et al. Capecitabine or folinic acid/fluorouracil i.v. bolus plus Eloxatin evaluation (COFFE trial) in metastatic colorectal carcinoma (MCRC): final results of the Southern Italy Cooperative Oncology Group (SICOG) phase III trial 0401. ECCO 14 The European Cancer Conference Barcelona 23‐27 September; 2007. 2007:248, abstr 3044.
De Gramont 2012 {published data only (unpublished sought but not used)}
-
- Gramont A, Cutsem E, Schmoll H‐J, Tabernero J, Clarke S, Moore MJ, et al. Bevacizumab plus oxaliplatin‐based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncology 2012;13:1225‐33. - PubMed
-
- Gramont A, Cutsem E, Tabernero J, Moore M, Cunningham D, Rivera F, et al. AVANT: results from a randomized, three‐arm multinational phase III study to investigate bevacizumab with either XELOX or FOLFOX4 versus FOLFOX4 alone as adjuvant treatment for colon cancer. Journal of Clinical Oncology 2011;29(Suppl 4):abstr 362.
De la Torre 2008 {published and unpublished data}
-
- Torre A, Garcia‐Berrocal MI, Arias F, Marino A, Valcarcel F, Magallon R, et al. Preoperative chemoradiotherapy for rectal cancer: randomized trial comparing oral uracil and tegafur and oral leucovorin vs. intravenous 5‐fluorouracil and leucovorin. International Journal of Radiation Oncology Biology Physics 2008;70(1):102‐10. - PubMed
Diaz‐Rubio 2007 {published data only (unpublished sought but not used)}
-
- Diaz‐Rubio E, Tabernero J, Gomez‐Espana A, Massuti B, Sastre J, Chaves M, et al. Phase III study of capecitabine plus oxaliplatin compared with continuous‐infusion fluorouracil plus oxaliplatin as first‐line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. Journal of Clinical Oncology 2007;25(27):4224‐30. - PubMed
Douillard 2002 {published data only (unpublished sought but not used)}
-
- Douillard JY, Hoff PM, Skillings JR, Eisenberg P, Davidson N, Harper P, et al. Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. Journal of Clinical Oncology 2002;20(17):3605‐16. - PubMed
Douillard 2014 {published and unpublished data}
-
- Douillard J‐Y, Zemelka T, Fountzilas G, Barone C, Schlichting M, Eggleton S, et al. Randomized phase II study evaluating UFOX plus cetuximab versus FOLFOX4 plus cetuximab as first‐line therapy in metastatic colorectal cancer: FUTURE. Annals of Oncology 2012;23(Suppl 4):iv5‐iv18, abstr 0‐0.0017. - PubMed
-
- Douillard JY, Zemelka T, Fountzilas G, Barone C, Schlichting M, Heighway J, et al. FOLFOX4 With Cetuximab vs. UFOX With Cetuximab as First‐Line Therapy in Metastatic Colorectal Cancer: The Randomized Phase II FUTURE Study. Clinical Colorectal Cancer 2014;13(1):14‐26. - PubMed
Ducreux 2011 {published data only (unpublished sought but not used)}
-
- Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, et al. Capecitabine plus oxaliplatin (XELOX) versus 5‐fluorouracil/leucovorin plus oxaliplatin (FOLFOX‐6) as first‐line treatment for metastatic colorectal cancer. Journal international du cancer [International Journal of Cancer] 2011;128:682‐90. - PubMed
-
- Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, et al. Efficacy and safety findings from a randomized phase III study of capecitabine (X) + oxaliplatin (O) (XELOX) vs. infusional 5‐FU/LV + O (FOLFOX‐6) for metastatic colorectal cancer (MCRC). Journal of Clinical Oncology 2007;25(18S):abstr 4029.
Ducreux 2013 {published and unpublished data}
-
- Ducreux M, Adenis A, Mendiboure J, Francois E, Boucher E, Chauffert M, et al. Efficacy and safety of bevacizumab (BEV)‐ based combination regimens in patients with metastatic colorectal cancer (mCRC): Randomized phase II study of BEV + FOLFIRI versus BEV + XELIRI (FNCLCC ACCORD 13/0503 study). Journal of Clinical Oncology 2009;27(15S):abstr 4086.
-
- Ducreux M, Adenis A, Pignon J‐P, François E, Chauffert B, Ichanté JL, et al. Efficacy and safety of bevacizumab‐based combination regimens in patients with previously untreated metastatic colorectal cancer: final results from a randomised phase II study of bevacizumab plus 5‐fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD13/0503 study). European Journal of Cancer 2013;49:1236‐45. - PubMed
-
- Malka D, Boige V, Jacques N, Vimond N, Adenis A, Boucher E, et al. Clinical value of circulating endothelial cell levels in metastatic colorectal cancer patients treated with first‐line chemotherapy and bevacizumab. Annals of Oncology 2012;23(4):919‐27. - PubMed
ECOG E5296 2012 {unpublished data only}
-
- Levy D, Leshne G, DiGiuseppe S, Bucher T. Analysis of E5296: phase III trial comparing a 28 day schedule of daily oral 5‐FU plus eniluracil to protracted intravenous infusion in previously untreated patients with advanced colorectal cancer. Boston (MA): Dana‐Farber Cancer Institute 2005 (Revised July 2, 2012):Technical Report No.: 1080E.
Fuchs 2007 {published and unpublished data}
-
- Fuchs CS, Marshall J, Barrueco. Capecitabine and irinotecan as first‐line treatment of advanced colorectal cancer‐ author reply. Journal of Clinical Oncology 2008;26(11):1908‐9. - PubMed
-
- Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first‐line treatment of metastatic colorectal cancer: results from the BICC‐C study. Journal of Clinical Oncology 2007;25(30):4779‐86. - PubMed
Hochster TREE‐1 2008 {published data only (unpublished sought but not used)}
-
- Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first‐line treatment of metastatic colorectal cancer: results of the TREE Study. Journal of Clinical Oncology 2008;26(21):3523‐9. - PubMed
Hochster TREE‐2 2008 {published data only (unpublished sought but not used)}
-
- Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first‐line treatment of metastatic colorectal cancer: results of the TREE Study. Journal of Clinical Oncology 2008;26(21):3523‐9. - PubMed
Hoff 2001 {published data only (unpublished sought but not used)}
-
- Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first‐line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. Journal of Clinical Oncology 2001;19(8):2282‐92. - PubMed
Hofheinz 2012 {published and unpublished data}
-
- Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non‐inferiority, phase 3 trial. Lancet Oncology 2012;13(6):579‐88. - PubMed
Kato 2012 {published and unpublished data}
-
- Gamoh M, Andoh H, Yamaguchi T, Maeda S, Sasaki Y, Suzuki T, et al. A randomized pilot study comparing safety and efficacy of irinotecan plus S‐1 plus bevacizumab (IRIS1BV) and modified FOLFIRI plus BV (mFOLFIRI+BV) in patients (pts) with metastatic colorectal cancer (MCRC): The result of T‐core0702. Annals of Oncology 2011;22(Suppl 9):ix49‐ix50, abstr 109.
-
- Kato S, Andoh H, Gamoh M, Yamaguchi T, Murakawa Y, Sasaki Y, et al. A randomized pilot study comparing safety and efficacy of irinotecan plus S‐1 (IRIS) plus bevacizumab (BV) and modified (m) FOLFIRI plus BV in patients (pts) with metastatic colorectal cancer (mCRC): first report of T‐CORE0702. Journal of Clinical Oncology 2011;4(Suppl):abstr 496.
-
- Kato S, Andoh H, Gamoh M, Yamaguchi T, Murakawa Y, Shimodaira H, et al. Safety verification trials of mFOLFIRI and sequential IRIS + bevacizumab as first‐ or second‐line therapies for metastatic colorectal cancer in Japanese patients. Oncology 2012;83:101‐7. - PubMed
Kim 2001a {published data only (unpublished sought but not used)}
-
- Kim NK, Lee KY, Park JK, Yun SH, Roh JK, Min JS. Prospective Randomized Trials Comparing Intravenous 5‐Fluorouracil and Oral Doxifluridine as a Postoperative Adjuvant Treatment for Advanced Rectal Cancer [Korean]. Journal of the Korean Surgical Society 2001;60(2):195‐9. - PubMed
-
- Min JS, Kim NK, Park JK, Yun SH, Noh JK. A prospective randomized trial comparing intravenous 5‐fluorouracil and oral doxifluridine as postoperative adjuvant treatment for advanced rectal cancer. Annals of Surgical Oncology 2000;7(9):674‐9. - PubMed
Kohne 2008 {published data only (unpublished sought but not used)}
-
- Kohne CH, Greve J, Hartmann JT, Lang I, Vergauwe P, Becker K, et al. Irinotecan combined with infusional 5‐fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first‐line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Annals of Oncology 2008;19(5):920‐6. - PubMed
Lembersky 2006 {published data only (unpublished sought but not used)}
-
- Lembersky BC, Wieand HS, Petrelli NJ, O'Connell MJ, Colangelo LH, Smith RE, et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project protocol C‐06. Journal of Clinical Oncology 2006;24(13):2059‐64. - PubMed
Martoni 2006 {published data only (unpublished sought but not used)}
-
- Martoni AA, Pinto C, Fabio F, Lelli G, Llimpe FLR, Gentile AL, et al. Capecitabine plus oxaliplatin (xelox) versus protracted 5‐fluorouracil venous infusion plus oxaliplatin (pvifox) as first‐line treatment in advanced colorectal cancer: a GOAM phase II randomised study (FOCA trial). European Journal of Cancer 2006;42(18):3161‐8. - PubMed
Mei 2014 {published data only}
-
- Mei Z, Yanhua Z, Weiyan G, Hongxia H, Lige Y, Tiandong K. Clinical observation of S‐1 plus oxaliplatin in the treatment of locally advanced or metastatic colorectal cancer [Chinese]. Cancer Research and Clinic 2014;26(12):820‐2.
Nogue 2005 {published data only (unpublished sought but not used)}
-
- Nogue M, Salud A, Batiste‐Alentorn E, Saigi E, Losa F, Cirera L, et al. Randomised study of tegafur and oral leucovorin versus intravenous 5‐fluorouracil and leucovorin in patients with advanced colorectal cancer. European Journal of Cancer 2005;41(15):2241‐9. - PubMed
-
- Salud A, Saigi E, Batiste‐Alentorn E, Losa F, Cirera L, Mendez M, et al. Randomized phase IV trial of oral tegafur and low dose leucovorin versus intravenous 5‐fluorouracil and leucovorin in the treatment of advanced colorectal cancer (ACC): final results. Journal of Clinical Oncology 2004;22(14S):abstr 3547.
Pectasides 2012 {published and unpublished data}
-
- Pectasides D, Papaxoinis G, Kalogeras K, Eleftheraki A, Xanthakis I, Makatsoris T, et al. XELIRI‐‐bevacizumab versus FOLFIRI‐bevacizumab as first‐line treatment in patients with metastatic colorectal cancer: a Hellenic Cooperative Oncology Group phase III trial with collateral biomarker analysis. BMC Cancer 2012;12(1):271. - PMC - PubMed
Pectasides 2015 {published data only (unpublished sought but not used)}
-
- Maniadakis N, Fragoulakis V, Pectasides D, Fountzilas G. XELOX versus FOLFOX6 as an adjuvant treatment in colorectal cancer: an economic analysis. Current Medical Research and Opinion 2009;25(3):797‐805. - PubMed
-
- Pectasides D, Karavasilis V, Papaxoinis G, Gourgioti G, Makatsoris T, Raptou G, et al. Randomized phase III clinical trial comparing the combination of capecitabine and oxaliplatin (CAPOX) with the combination of 5‐fluorouracil, leucovorin and oxaliplatin (modified FOLFOX6) as adjuvant therapy in patients with operated high‐risk stage II or stage III colorectal cancer. BMC Cancer 2015;15:384. - PMC - PubMed
-
- Pectasides DG, Papaxoinis G, Xanthakis I, Kouvatseas G, Kotoula V, Xiros N, et al. Randomized phase III trial of FOLFOX versus XELOX as adjuvant chemotherapy in patients with early‐stage colorectal adenocarcinoma. Journal of Clinical Oncology 2014;32(5s):abstr 3617.
Porschen 2007 {published data only (unpublished sought but not used)}
-
- Arkenau HT, Arnold D, Cassidy J, Diaz‐Rubio E, Douillard JY, Hochster H, et al. Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. Journal of Clinical Oncology 2008;26(36):5910‐7. - PubMed
-
- Porschen R, Arkenau HT, Kubicka S, Greil R, Seufferlein T, Freier W, et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. Journal of Clinical Oncology 2007;25(27):4217‐23. - PubMed
Rothenberg 2008 {published data only (unpublished sought but not used)}
-
- Rothenberg M, Navarro M, Butts C, Bang Y, Cox J, Goel R, et al. Phase III trial of capecitabine + oxaliplatin (XELOX) vs. 5‐fluorouracil (5‐FU), leucovorin (LV), and oxaliplatin (FOLFOX4) as 2nd‐line treatment for patients with metastatic colorectal cancer (MCRC). Journal of Clinical Oncology 2007;25(18S):abstr 4031.
-
- Rothenberg ML, Cox JV, Butts C, Navarro M, Bang YJ, Goel R, et al. Capecitabine plus oxaliplatin (XELOX) versus 5‐fluorouracil/folinic acid plus oxaliplatin (FOLFOX‐4) as second‐line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Annals of Oncology 2008;19(10):1720‐6. - PubMed
Schilsky 2002a {published and unpublished data}
-
- Schilsky RL, Levin J, West WH, Wong A, Colwell B, Thirlwell MP, et al. Randomized, open‐label, phase III study of a 28‐day oral regimen of eniluracil plus fluorouracil versus intravenous fluorouracil plus leucovorin as first‐line therapy in patients with metastatic/advanced colorectal cancer. Journal of Clinical Oncology 2002;20(6):1519‐26. - PubMed
Seymour 2011 {published data only (unpublished sought but not used)}
Shigeta 2016 {published and unpublished data}
-
- Hasegawa H, Endo T, Ochiai D, Uchida H, Okabayashi T, Imai S, et al. A randomized Phase II study of tegafur/uracil (UFT), oral leucovorin and irinotecan, combination therapy (TEGAFIRI)+bevacizumab versus FOLFIRI+bevacizumab for the first‐line treatment of patients with metastatic colorectal cancer. European Journal of Cancer 2013;49 (Suppl 2):S557, abstr 2390.
-
- Shigeta K, Hasegawa H, Okabayashi K, Tsuruta M, Ishii Y, Endo T, et al. Randomized phase II trial of TEGAFIRI (tegafur/uracil, oral leucovorin, irinotecan) compared with FOLFIRI (folinic acid,5‐fluorouracil, irinotecan) in patients with unresectable/recurrent colorectal cancer. International Journal of Cancer 2016;139(4):946‐54. - PubMed
Shimada 2014 {published data only (unpublished sought but not used)}
-
- Shimada Y, Hamaguchi T, Mizusawa J, Saito N, Kanemitsu Y, Takiguchi N, et al. Randomised phase III trial of adjuvant chemotherapy with oral uracil and tegafur plus leucovorin versus intravenous fluorouracil and levofolinate in patients with stage III colorectal cancer who have undergone Japanese D2/D3 lymph node dissection: Final results of JCOG0205. European Journal of Cancer 2014;50(13):2231‐2240. - PubMed
-
- Shimada Y, Hamaguchi T, Moriya Y, Saito N, Kanemitsu Y, Takiguchi N, et al. Randomized phase III study of adjuvant chemotherapy with oral uracil and tegafur plus leucovorin versus intravenous fluorouracil and levofolinate in patients (pts) with stage III colon cancer (CC): final results of Japan Clinical Oncology Group study (JCOG0205). Journal of Clinical Oncology 2012;30(Suppl):abstr 3524.
Silvestris 2010 {published data only (unpublished sought but not used)}
-
- Silvestris N, Maiello E, Vita F, Cinieri S, Santini D, Russo A, et al. Update on capecitabine alone and in combination regimens in colorectal cancer patients. Cancer Treatment Reviews 2010;36(Suppl 3):S46‐55. - PubMed
Souglakos 2012 {published and unpublished data}
-
- Souglakos J, Ziras N, Kakolyris S, Boukovinas I, Kentepozidis N, Makrantonakis P, et al. Randomised phase‐II trial of CAPIRI (capecitabine, irinotecan) plus bevacizumab vs FOLFIRI (folinic acid, 5‐fluorouracil, irinotecan) plus bevacizumab as first‐line treatment of patients with unresectable/metastatic colorectal cancer (mCRC). British Journal of Cancer 2012;106(3):453‐9. - PMC - PubMed
Twelves 2012 {published and unpublished data}
-
- Scheithauer W, McKendrick J, Begbie S, Borner M, Burns WI, Burris HA, et al. Oral capecitabine as an alternative to i.v. 5‐fluorouracil‐based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Annals of Oncology 2003;14(12):1735‐43. - PubMed
-
- Twelves C, Scheithauer W, McKendrick J, Seitz JF, Hazel G, Wong A, et al. Capecitabine versus 5‐fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X‐ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Annals of Oncology 2012;23(5):1190‐7. - PubMed
-
- Twelves C, Wong A, Nowacki MP, Abt M, Burris H, 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. New England Journal of Medicine 2005;352(26):2696‐704. - PubMed
Van Cutsem 2001a {published and unpublished data}
-
- Cutsem E, Sorensen J, Cassidy J, Daniel F, Harper P, Bailey N, et al. International phase III study of oral eniluracil (EU) plus 5‐fluorouracil (5‐FU) versus intravenous (IV) 5‐FU plus leucovorin (LV) in the treatment of advanced colorectal cancer (ACC). Journal of Clinical Oncology, Proceedings of American Society of Clinical Oncology 2001;20:abstr 522.
Van Cutsem 2001b {published data only (unpublished sought but not used)}
-
- Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. Journal of Clinical Oncology 2001;19(21):4097‐106. - PubMed
Yamada 2013 {published and unpublished data}
-
- Matsumoto H, Yamada Y, Takahari D, Baba H, Yoshida K, Komatsu Y, et al. The SOFT study: A randomized phase III trial of S‐1/oxaliplatin (SOX)plus bevacizumab versus 5‐FU/l‐LV/oxaliplatin (mFOLFOX6) plus bevacizumab in patients with metastatic colorectal cancer [SOFT Study Group]. European Journal of Cancer 2013;49(Suppl 2):S486, abstr 2167.
-
- Takahari D, Yamada Y, Matsumoto H, Baba H, Yoshida K, Nakamura M, et al. A randomized phase III trial of S‐1/oxaliplatin (SOX) plus bevacizumab versus 5‐FU/l‐LV/oxaliplatin (mFOLFOX6) plus bevacizumab in patients with metastatic colorectal cancer: the SOFT study. Journal of Clinical Oncology 2013;15(suppl):abstr 3519.
-
- Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S‐1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open‐label, non‐inferiority, randomised phase 3 trial. Lancet Oncology 2013;14:1278‐86. - PubMed
Yamazaki 2015 {published and unpublished data}
-
- Ojima H, Yamakazi K, Kuwano H, Otsuji T, Kato T, Shimada K, et al. Randomized Phase II study of S‐1, oral leucovorin, and oxaliplatin combination therapy (SOL) versus mFOLFOX6 in patients with untreated metastatic colorectal cancer (mCRC). European Journal of Cancer 2011;47(Suppl 1):427, abstr 6118.
-
- Otsuji T, Yamazaki K, Ojima H, Kuwano H, Kato T, Shimada K, et al. Updated survival results of the randomized phase II study of S‐1, oral leucovorin, and oxaliplatin combination therapy (SOL) versus mFOLFOX6 in patients with untreated metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2012;30(Suppl 4):abstr 586.
-
- Yamazaki K, Kuwano H, Ojima H, Otsuji T, Kato T, Shimada K, et al. A randomized phase II study of combination therapy with S‐1, oral leucovorin, and oxaliplatin (SOL) and mFOLFOX6 in patients with previously untreated metastatic colorectal cancer. Cancer Chemotherapy and Pharmacology 2015;75(3):569‐77. - PubMed
Yasui 2015 {published and unpublished data}
-
- Baba H, Muro K, Yasui H, Shimada Y, Tsuji A, Sameshima S, et al. Updated results of the FIRIS study: A phase II/III trial of 5‐FU/l‐leucovorin/irinotecan (FOLFIRI) versus irinotecan/S‐1 (IRIS) as second‐line chemotherapy for metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2011;29(Suppl):abstr 3562.
-
- Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, et al. Irinotecan plus S‐1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second‐line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non‐inferiority study (FIRIS study). Lancet Oncology 2010;11(9):853‐60. - PubMed
-
- Yasui H, Muro K, Shimada Y, Tsuji A, Sameshima S, Baba H, et al. A phase 3 non‑inferiority study of 5‑FU/l‑leucovorin/irinotecan(FOLFIRI) versus irinotecan/S‑1 (IRIS) as second‑line chemotherapy for metastatic colorectal cancer: updated results of the FIRIS study. Journal of Cancer Research and Clinical Oncology 2015;141:153‐60. - PMC - PubMed
Yu 2005 {published data only (unpublished sought but not used)}
-
- Yu B‐M, Wu W‐Q. Irinotecan combined with fluoropyrimidine in treatment for advanced/metastatic colorectal carcinoma. Chung‐Hua Wai Ko Tsa Chih [Chinese Journal of Surgery] 2005;43(9):557‐60. - PubMed
References to studies excluded from this review
Ansfield 1977 {published data only}
-
- Ansfield F, Klotz J, Nealon T, Ramirez G, Minton J, Hill G, et al. A phase III study comparing the clinical utility of four regimens of 5‐fluorouracil: a preliminary report. Cancer 1977;39(1):34‐40. - PubMed
Bajetta 1997 {published data only}
-
- Bajetta E, Bartolomeo M, Somma L, Vecchio M, Artale S, Zunino F, et al. Doxifluridine in colorectal cancer patients resistant to 5‐fluorouracil (5‐FU) containing regimens. European Journal of Cancer Part A 1997;33(4):687‐90. - PubMed
Bedikian 1983 {published data only}
-
- Bedikian AY, Stroehlein J, Korinek J, Karlin D, Bodey GP. A comparative study of oral tegafur and intravenous 5‐fluorouracil in patients with metastatic colorectal cancer. American Journal of Clinical Oncology 1983;6(2):181‐6. - PubMed
Bjerkeset 1986 {published data only}
-
- Bjerkeset T, Fjosne HE. Comparison of oral ftorafur and intravenous 5‐fluorouracil in patients with advanced cancer of the stomach, colon or rectum. Oncology 1986;43(4):212‐5. - PubMed
Borner 2002 {published data only}
-
- Borner MM, Schoffski P, Wit R, Caponigro F, Comella G, Sulkes A, et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. European Journal of Cancer 2002;38(3):349‐58. - PubMed
Douglass 1978 {published data only}
-
- Douglass HO, Jr, Lavin PT, Woll J, Conroy JF, Carbone P. Chemotherapy of advanced measurable colon and rectal carcinoma with oral 5‐fluorouracil, alone or in combination with cyclophosphamide or 6‐thioguanine, with intravenous 5‐fluorouracil or beta‐2'‐deoxythioguanosine or with oral 3(4‐methyl‐cyclohexyl)‐1(2‐chlorethyl)‐1‐nitrosourea: a Phase II‐III study of the Eastern Cooperative Oncology Group (EST 4273). Cancer 1978;42(6):2538‐45. - PubMed
Fan 2005 {published data only}
Hahn 1975 {published data only}
-
- Hahn RG, Moertel CG, Schutt AJ, Bruckner HW. A double‐blind comparison of intensive course 5‐flourouracil by oral vs. intravenous route in the treatment of colorectal carcinoma. Cancer 1975;35(4):1031‐5. - PubMed
Hennig 2008 {published data only}
-
- Hennig IM, Naik JD, Brown S, Szubert A, Anthoney DA, Jackson DP, et al. Severe sequence‐specific toxicity when capecitabine is given after Fluorouracil and leucovorin. Journal of Clinical Oncology 2008;26(20):3411‐7. - PubMed
Kim 2001b {published data only (unpublished sought but not used)}
-
- Kim NK, Min JS, Park JK, Yun SH, Sung JS, Jung HC, et al. Intravenous 5‐fluorouracil versus oral doxifluridine as preoperative concurrent chemoradiation for locally advanced rectal cancer: prospective randomized trials. Japanese Journal of Clinical Oncology 2001;31(1):25‐9. - PubMed
Lima 2005 {published data only}
-
- Lima APR, Giglio A. Randomized crossover trial of intravenous 5‐FU versus oral UFT both modulated by leucovorin: a one‐centre experience. European Journal of Cancer Care 2005;14(2):151‐4. - PubMed
Maetani 1993 {published data only}
-
- Maetani S. Multicenter clinical trial of UFT with FT for patients with colorectal cancer. Therapeutic Research 1993;14(6):226‐32.
Munoz 2008 {published data only (unpublished sought but not used)}
-
- Munoz Llarena A, Salud Salvia A, García Girón C, Murias Rosales A, Burillo M, Arizcun Sánchez‐Morate A, et al. Randomized phase III trial, with irinotecan and capecitabine (XELIRI) versus irinotecan, 5FU and folinic acid (Saltz regimen) in first‐line treatment on metastatic colorectal cancer patients (CRC). ASCO 2008 Gastrointestinal Cancers Symposium. 2008:abstr 379.
NCT00070122 {published and unpublished data}
-
- NCT00070122. Combination chemotherapy and bevacizumab in treating patients with locally recurrent colorectal cancer. http://clinicaltrials.gov/ct2/show/NCT00070122 (accessed 8 June 2016).
NCT01193452 {published and unpublished data}
-
- NCT01193452. S‐1/leucovorin (SL) versus sLV5FU2 as the first‐line treatment for elderly patients with colorectal cancer. http://clinicaltrials.gov/ct2/show/NCT01193452 (accessed 8 June 2016).
NCT01196260 {published data only}
-
- NCT01196260. Combination chemotherapy treatments in patients with colorectal cancer stage II and III. http://clinicaltrials.gov/ct2/show/NCT01196260 (accessed 8 June 2016).
NCT01279681 {published data only}
-
- NCT01279681. Randomized phase III trial of mFOLFOX7 or XELOX plus bevacizumab versus 5‐fluorouracil/leucovorin or capecitabine plus bevacizumab as first‐line treatment in elderly patients with metastatic colorectal cancer. http://clinicaltrials.gov/ct2/show/NCT01279681 (accessed 8 June 2016).
NCT01736904 {published data only (unpublished sought but not used)}
-
- NCT01736904. wXELIRI versus FOLFIRI regimen in the treatment of advanced colorectal cancer patients. http://clinicaltrials.gov/ct2/show/NCT01736904 (accessed 8 June 2016).
Pfeiffer 2006 {published data only}
-
- Pfeiffer P, Mortensen JP, Bjerregaard B, Eckhoff L, Schonnemann K, Sandberg E, et al. Patient preference for oral or intravenous chemotherapy: A randomised cross‐over trial comparing capecitabine and Nordic fluorouracil/leucovorin in patients with colorectal cancer. European Journal of Cancer 2006;42(16):2738‐43. - PubMed
Queißer 1979 {published data only}
-
- Queißer W, Schaefer J, Arnold H, Drings P, Geldmacher J, Hartwich G, et al. A prospective multi‐centre study of the response of metastatic gastrointestinal tumours [Prospektive kontrollierte studie bei metastasierten gastrointestinalen tumoren: vergleich zwischen 5‐fluorouracil ‐ carmustin und ftorafur ‐ carmustin]. Deutsche Medizinische Wochenschrift 1979;104(35):1231‐6. - PubMed
Revazishvili 2008 {published data only (unpublished sought but not used)}
-
- Revazishvili P, Shavdia M, Chvamichva R, Shavidia N. Oral ftorafur in treatment of advanced colorectal cancer. Annals of Oncology 2008;19(Suppl 8):viii147, abstr 437.
Sizer 2006 {published data only (unpublished sought but not used)}
-
- Sizer B, Makris A, Barone C, Mainwaring P, Eggleton P. QoL and resource use analysis of tegafur‐uracil/LV or 5‐FU/LV in first‐line metastatic colorectal cancer (mCRC): final results of a multicenter phase II study. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I 2006;24(18S):abstr 3631.
Skof 2009 {published data only (unpublished sought but not used)}
-
- Skof E, Rebersek M, Hlebanja Z, Ocvirk J. Capecitabine plus Irinotecan (XELIRI regimen) compared to 5‐FU/LV plus Irinotecan (FOLFIRI regimen) as neoadjuvant treatment for patients with unresectable liver‐only metastases of metastatic colorectal cancer: a randomised prospective phase II trial. BMC Cancer 2009;9:120. - PMC - PubMed
Tournigand 2012 {published data only}
-
- Tournigand C, Samson B, Scheithauer W, Lledo G, Viret F, Andre T, et al. Bevacizumab (Bev) with or without erlotinib as maintenance therapy, following induction first‐line chemotherapy plus Bev, in patients (pts) with metastatic colorectal cancer (mCRC): Efficacy and safety results of the International GERCOR DREAM phase III trial. Abstract No: LBA3500, Presenter: Christophe Tournigand, MD, PhD. 2012 ASCO Annual Meeting, Gastrointestinal (Colorectal) Cancer Track‐ Oral Abstract Session. Available from: http://www.asco.org/ASCOv2/MultiMedia/Virtual+Meeting?&vmview=vm_ses... (accessed 26 September 2012).
-
- Tournigand C, Samson B, Scheithauer W, Lledo G, Viret F, Andre T, et al. Bevacizumab (Bev) with or without erlotinib as maintenance therapy, following induction first‐line chemotherapy plus Bev, in patients (pts) with metastatic colorectal cancer (mCRC): Efficacy and safety results of the International GERCOR DREAM phase III trial. Journal of Clinical Oncology 2012;30(Suppl):abstr LBA3500.
Twelves 2006 {published data only}
-
- Twelves C, Gollins S, Grieve R, Samuel L. A randomised cross‐over trial comparing patient preference for oral capecitabine and 5‐fluorouracil/leucovorin regimens in patients with advanced colorectal cancer. Annals of Oncology 2006;17(2):239‐45. - PubMed
References to ongoing studies
Barsukov 2015 {published data only}
-
- Barsukov Y, Perevoshikov A, Kuzmichev D, Mammadli Z, Polynovskiy A. Combined treatment of resectable distal rectal cancer with different fluoropyrimidines. European Journal of Surgical Oncology 2012;38(9):832, abstr 324.
GOIM 2802 {unpublished data only}
-
- GOIM 2802. Bevacizumab + FOLFOX4 or XELOX2 as first‐line treatment in colorectal cancer. Randomized phase II study. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2010‐022091‐31/IT (accessed 29 August 2016).
Joarder 2012 {published data only}
-
- Joarder R, Basu A, Choudhury KB, Dasgupta C, Dasgupta P, Ghosh K. Neoadjuvant chemoradiation with oral capecitabine versus intravenous 5‐fluorouracil and leucovorin in locally advanced carcinoma rectum – a randomized trial: an interim report. Journal of Cancer Research and Therapeutics 2012;8(Suppl 3):S159.
Muro 2016 {published data only}
-
- Muro K, Kim TW, Park YS, Uetake H, Nishina T, Nozawa H, et al. A multinational, randomized, phase III trial of XELIRI (+bevacizumab) versus FOLFIRI (+bevacizumab) as the second‐line chemotherapy for metastatic colorectal cancer: Asian XELIRI project (AXEPT). Journal of Clinical Oncology 2016;34(Suppl 4S):abstr TPS780. - PMC - PubMed
NCT02280070 {published data only}
-
- NCT02280070. RP II study of SOX vs mFOLFOX6 in patients with resectable rectal cancer (KSCC1301). https://clinicaltrials.gov/ct2/show/record/NCT02280070 (accessed 27 June 2016).
Additional references
Allegra 2011
André 2009
-
- André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. Journal of Clinical Oncology 2009;27(19):3109‐16. - PubMed
Arkenau 2008
-
- Arkenau HT, Arnold D, Cassidy J, Diaz‐Rubio E, Douillard JY, Hochster H, et al. Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. Journal of Clinical Oncology 2008;26(36):5910‐7. - PubMed
Bennouna 2013
-
- Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncology 2013;14(1):29‐37. - PubMed
Benson 2004
-
- Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. Journal of Clinical Oncology 2004;22(16):3408‐19. - PubMed
Blum 1999
-
- Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, et al. Multicenter Phase II study of capecitabine in paclitaxel‐refractory metastatic breast cancer. Journal of Clinical Oncology 1999;17(2):485‐93. - PubMed
Bosset 2006
-
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic‐Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. New England Journal of Medicine 2006;355(11):1114‐23. - PubMed
Braun 2011
Brenner 2014
-
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383(9927):1490‐502. - PubMed
Bujko 2006
-
- Bujko K, Nowacki MP, Nasierowska‐Guttmejer A, Michalski W, Bebenek M, Kryj M. Long‐term results of a randomized trial comparing preoperative short‐course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. British Journal of Surgery 2006;93(10):1215‐23. - PubMed
Cafiero 2003
-
- Cafiero F, Gipponi M, Lionetto R, P.A.R. Cooperative Study Group. Randomised clinical trial of adjuvant postoperative RT vs. sequential postoperative RT plus 5‐FU and levamisole in patients with stage II‐III resectable rectal cancer: a final report. Journal of Surgical Oncology 2003;83(3):140‐6. - PubMed
Calabresi 1991
-
- Calabresi F, Repetto M. Doxifluridine in advanced colorectal cancer. Journal of Surgical Oncology Supplement 1991;2:124‐8. - PubMed
Cassidy 2002
-
- Cassidy J, Twelves C, Cutsem E, Hoff P, Bajetta E, Boyer M, et al. First‐line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5‐fluorouracil/leucovorin. Annals of Oncology 2002;13(4):566‐75. - PubMed
Cassidy 2005
-
- Cassidy J. Benefits and drawbacks of the use of oral fluoropyrimidines as single‐agent therapy in advanced colorectal cancer. Clinical Colorectal Cancer 2005;5(Suppl 1):S47‐50. - PubMed
Cassidy 2008
-
- Cassidy J, Clarke S, Diaz‐Rubio E, Scheithauer W, Figer A, Wong R, et al. XELOX vs. FOLFOX4: Update of efficacy results from XELOX‐1/NO16966, a randomized phase III trial of first‐line treatment for patients (pts) with metastatic colorectal cancer (MCRC). ASCO 2008 Gastrointestinal Cancer Symposium. 2008:abstr 341.
Cassidy 2011b
-
- Cassidy J, Saltz L, Twelves C, Cutsem E, Hoff P, Kang Y, et al. Efficacy of capecitabine versus 5‐fluorouracil in colorectal and gastric cancers: a meta‐analysis of individual data from 6171 patients. Annals of Oncology 22;12:2604‐9. - PubMed
Chandler 2013
-
- Chandler J, Churchill R, Higgins J, Lasserson T, Tovey D. Methodological standards for the conduct of new Cochrane Intervention Reviews. Version 2.3. 02 December 2013. Available from: http://editorial‐unit.cochrane.org/mecir.
Chau 2009
Cheng 2014
-
- Cheng AL, Li J, Vaid AK, Ma BB, Teh C, Ahn JB, et al. Adaptation of international guidelines for metastatic colorectal cancer: an asian consensus. Clinical Colorectal Cancer 2014;13(3):145‐55. - PubMed
Chionh 2010
Chuah 2011
-
- Chuah B, Goh BC, Lee SC, Soong R, Lau F, Mulay M, et al. Comparison of the pharmacokinetics and pharmacodynamics of S‐1 between Caucasian and East Asian patients. Cancer Science 2011;102(2):478‐83. - PubMed
Cuppone 2008
-
- Cuppone F, Bria E, Maio M, Carlini P, Sperduti I, Milella M, et al. Meta‐analysis of randomized clinical trials (RCTS) exploring capecitabine (C) versus 5‐fluorouracil (FU) in combination with oxaliplatin (OX) as 1st‐line chemotherapy for metastatic colorectal cancer (MCRC). Annals of Oncology 2008;19(Suppl 8):viii131, abstr 380P.
Deeks 2011
-
- Deeks J, Higgins J, Altman D, on behalf of the Cochrane Statistical Methods Group (Editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins J, Green S (Editors).Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dencausse 2002
-
- Dencausse Y, Hartung G, Sturm J, Kopp‐Schneider A, Hagmüller E, Wojatschek C, et al. Adjuvant chemotherapy in stage III colon cancer with 5‐fluorouracil and levamisole versus 5‐fluorouracil and leucovorin. Onkologie 2002;25(5):426‐30. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled clinical trials 1986;7(3):177‐88. - PubMed
Douillard 2010
-
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first‐line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. Journal of Clinical Oncology 2010;28(31):4697‐705. - PubMed
Edge 2009
-
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A 3rd (Editors). Chapter 14: Colon and Rectum. AJCC Cancer staging manual. 7th Edition. Springer, 2009:173‐206.
EMA 2016
-
- Xeloda (capecitabine) . European Medicines Agency 2016, updated 26 July 2016. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (accessed 12 September 2016).
FDA 2010
-
- U.S. Department of Health and Human Services, Food, Drug Administration. Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for Industry‐ Non‐inferiority Clinical Trials. FDA 2010.
Ferlay 2013
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Globocan 2012 v1.0, Cancer Incidence and Mortality Worldwide, IARC CancerBase No. 11 [Internet]. International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr (accessed on 10 March 2016).
Figueredo 2008
Francini 1994
-
- Francini G, Petrioli R, Lorenzini L, Mancini S, Armenio S, Tanzini G, et al. Folinic acid and 5‐fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology 1994;106(4):899‐906. - PubMed
Gerard 2006
-
- Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon‐Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3‐4 rectal cancers: results of FFCD 9203. Journal of Clinical Oncology 2006;24(28):4620‐5. - PubMed
Giantonio 2007
-
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. Journal of Clinical Oncology 2007;25(12):1539‐44. - PubMed
Gill 2004
-
- Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil‐based adjuvant therapy for stage II and III colon cancer: who benefits and by how much?. Journal of Clincal Oncology 2004;22(10):1797‐806. - PubMed
GRADEpro [Computer program] [Computer program]
-
- Hamilton (ON): GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Grage 1981
-
- Grage TB, Moss SE. Adjuvant chemotherapy in cancer of the colon and rectum: demonstration of effectiveness of prolonged 5‐FU chemotherapy in a prospectively controlled, randomized trial. The Surgical clinics of North America 1981;61(6):1321‐9. - PubMed
Gray 2007
-
- Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. The Lancet 2007;370(9604):2020‐9. - PubMed
Guyatt 2008a
Guyatt 2008b
Haller 2005
-
- Haller DG, Catalano PJ, Macdonald JS, O'Rourke MA, Frontiera MS, Jackson DV, et al. Phase III study of fluorouracil, leucovorin and levamisole in high‐risk stage II and III colon cancer: final report of Intergroup 0089. Journal of Clinical Oncology 2005;23(34):8671‐8. - PubMed
Haller 2008
-
- Haller DG, Cassidy J, Clarke SJ, Cunningham D, Cutsem E, Hoff PM, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. Journal of Clinical Oncology 2008;26(13):2118‐23. - PubMed
Haller 2011
-
- Haller DG, Tabernero J, Maroun J, Braud F, Price T, Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. Journal of Clinical Oncology 2011;29(11):1465‐71. - PubMed
Hamaguchi 2015
-
- Hamaguchi T, Shimada Y, Mizusawa J. Kinugasa Y, Kanemitsu Y, Ohue M, et al. Randomized phase III study of adjuvant chemotherapy with S‐1 versus capecitabine (cape) in patients with stage III colon cancer (CC): Results of Japan Clinical Oncology Group study (JCOG0910). Journal of Clinical Oncology 2015;33(Suppl):abstr 3512.
Higgins 2011a
-
- Higgins J, Altman DG, Sterne J on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins J, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins J, Deeks J, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. Higgins J, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011.
Higgins 2011c
-
- Deeks J, Higgins J and Altman DJ on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. Higgins J, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org: The Cochrane Collaboration, 2011, The Cochrane Collaboration, 2011.
Hoff 2000
-
- Hoff PM. The tegafur‐based dihydropyrimidine dehydrogenase inhibitory fluoropyrimidines, UFT/leucovorin (ORZEL) and S‐1: a review of their clinical development and therapeutic potential. Investigational New Drugs 2000;18(4):331‐42. - PubMed
Hong 2012
-
- Hong YS, Park YS, Lim HY, Lee J, Kim TW, Kim K‐P, et al. S‐1 plus oxaliplatin versus capecitabine plus oxaliplatin for first‐line treatment of patients with metastatic colorectal cancer: a randomised, non‐inferiority phase 3 trial. Lancet Oncology 2012;13:1125‐32. - PubMed
Hurwitz 2004
-
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine 2004;350(23):2335‐42. - PubMed
IMPACT Investigators 1995
-
- International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. The Lancet 1995;345(8955):939‐44. - PubMed
Kwakman 2017
-
- Kwakman JJM, Simkens LHJ, Rooijen JM, Wouw AJ, Loosveld OJL, Creemers GJM, et al. Randomised phase 3 study of S‐1 versus capecitabine, with bevacizumab optional in both arms, in the first‐line treatment of metastatic colorectal cancer (mCRC), the SALTO study of the Dutch Colorectal Cancer Group. European Journal of Cancer 2017;71 (Supplement 1):S5.
Laurie 1989
-
- Laurie JA, Moertel CG, Fleming TR, Wieand HS, Leigh JE, Rubin J, et al. Surgical adjuvant therapy of large‐bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. Journal of Clinical Oncology 1989;7(10):1447‐56. - PubMed
Lenz 2015
Liu 1997
-
- Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. Journal of Clinical Oncology 1997;15(1):110‐5. - PubMed
Lucas 2011
-
- Lucas AS, O'Neil BH, Goldberg RM. A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clinical Colorectal Cancer 2011;10(4):238‐44. - PubMed
Malet‐Martino 2002
-
- Malet‐Martino M, Martino R. Clinical studies of three oral prodrugs of 5‐fluorouracil (capecitabine, UFT, S‐1): a review. Oncologist 2002;7(4):288‐323. - PubMed
Mayer 2015
-
- Mayer RJ, Cutsem E, Falcone A, Yoshino T, Garcia‐Carbonero R, Mizunuma N, et al. Randomized trial of TAS‐102 for refractory metastatic colorectal cancer. The New England Journal of Medicine 2015;372(20):1909‐19. - PubMed
Meta‐analysis Group in Cancer 1998a
-
- Meta‐analysis Group in Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Journal of Clinical Oncology 1998;16:301‐8. - PubMed
Meta‐analysis Group in Cancer 1998b
-
- Meta‐analysis Group in Cancer. Toxicity of fluorouracil in patients with advanced colorectal cancer: Effect of administration schedule and prognostic factors. Journal of Clinical Oncology 1998;16:3537‐41. - PubMed
Midgley 2009
-
- Midgley R, Kerr DJ. Capecitabine: have we got the dose right?. Nature Clinical Practice Oncology 2009;6(1):17‐24. - PubMed
Miyamoto 2014
-
- Miyamoto Y, Sakamoto Y, Yoshida N, Baba H. Efficacy of S‐1 in colorectal cancer. Expert Opinion on Pharmacotherapy 2014;15(12):1761‐70. - PubMed
Moertel 1990
-
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon cancer. New England Journal of Medicine 1990;322(6):352‐8. - PubMed
Montagnani 2010
-
- Montagnani F, Chiriatti A, Licitra S, Aliberti C, Fiorentini G. Differences in efficacy and safety between capecitabine and infusional 5‐fluorouracil when combined with irinotecan for the treatment of metastatic colorectal cancer. Clinical Colorectal Cancer 2010;9(4):243‐7. - PubMed
NCCN 2016
-
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Colon Cancer (Version 2.2016). https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed 14 September 2016).
Nordic 1992
-
- Nordic Gastrointestinal Tumor Adjuvant Therapy Group. Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. Journal of Clinical Oncology 1992;10(6):904‐11. - PubMed
O'Connell 1997
-
- O'Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, et al. Controlled trial of fluorouracil and low‐dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. Journal of Clinical Oncology 1997;15(1):246‐50. - PubMed
O'Connell 1998
-
- O'Connell MJ, Laurie JA, Kahn M, Fitzgibbons RJ Jr, Erlichman C, Shepherd L, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high‐risk colon cancer. Journal of Clinical Oncology 1998;16(1):295‐300. - PubMed
Pazdur 2016
-
- Pazdur R. Cancer drug information: FDA approval for capecitabine. National Cancer Institute at the National Institutes of Health 2012, updated 2 July 2013. Available from: http://www.cancer.gov/cancertopics/druginfo/fda‐capecitabine (accessed 13 September 2016).
Peeters 2010
-
- Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second‐line treatment in patients with metastatic colorectal cancer. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology 2010;28(31):4706‐13. - PubMed
Petrelli 2012
-
- Petrelli F, Cabiddu M, Barni S. 5‐Fluorouracil or capecitabine in the treatment of advanced colorectal cancer: a pooled‐analysis of randomized trials. Medical Oncology 2012;29(2):1020‐9. - PubMed
Punt 2008
-
- Punt CJ, Koopman M. Capecitabine and irinotecan as first‐line treatment of advanced colorectal cancer. Journal of Clinical Oncology 2008;26(11):1907‐8; author reply 8‐9. - PubMed
Quasar 2007
-
- Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, WilliamsNS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370(9604):2020‐9. - PubMed
RevMan [Computer Program] [Computer program]
-
- Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saletti 2008
-
- Saletti P, Sessa C, Cavalli F. Commentary 2008: 25 or 50 years later?. Journal of Clinical Oncology 26;30:4854‐5. - PubMed
Sargent 2009
Sasse 2009a
-
- Sasse AD, Sasse EC, dos Santos LV, Lima JS, Nascente CM, Saito HP, et al. Oral fluoropyrimidines versus 5‐fluorouracil for colorectal cancer: Results of a systematic review and meta‐analysis. Journal of Clinical Oncology 2009;27(15s):abstr4111.
Sasse 2009b
-
- Sasse AD, Sasse EC, dos Santos LV, Lima JS, Nascente CM, Saito HP, et al. Oral fluoropyrimidines versus 5‐fluorouracil for colorectal cancer: Results of a systematic review and meta‐analysis. Abstract No: 4111, Andre D Sasse, MD, PhD. 2009 ASCO Annual Meeting, Gastrointestinal (Colorectal) Cancer Track‐ General Poster Session. http://www.asco.org/ASCOv2/MultiMedia/Virtual+Meeting?&vmview=vm_ses... (accessed 26 September 2012).
Scheithauer 1993
Schilsky 2002b
-
- Schilsky RL. Inhibiting 5‐fluorouracil breakdown: a broken down approach to 5‐fluorouracil modulation. Clinical Colorectal Cancer 2002;2(1):51‐2. - PubMed
Schmoll 2007
-
- Schmoll HJ, Cartwright T, Tabernero J, Nowacki MP, Figer A, Maroun J, et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1,864 patients. Journal of Clinical Oncology 2007;25(1):102‐9. - PubMed
Schmoll 2010
-
- Schmoll HJ. S‐1 for advanced colorectal cancer: do we need another oral fluorouracil prodrug?. Lancet Oncology 2010;11(9):808‐9. - PubMed
Schmoll 2014
-
- Schmoll HJ, Twelves C, Sun W, O'Connell MJ, Cartwright T, McKenna E, et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post‐relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncology 2014;15(13):1481‐92. - PMC - PubMed
Schünemann 2011
-
- Schünemann H, Oxman A, Vist G, Higgins J, Deeks J, Glasziou P, et al on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins J, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Siegel 2017
-
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. Ca: A Cancer Journal for Clinicians, 2017 Mar 1;doi: 10.3322/caac.21395:[Epub ahead of print]. - PubMed
Sterne 2011
-
- Sterne J, Egger M, Moher D, on behalf of the Cochrane Bias Methods Group (Editors). Chapter 10: Addressing reporting bias. In: Higgins J, Green S (Editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Taal 2001
Tabernero 2015
-
- Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia‐Carbonero R, et al. Ramucirumab versus placebo in combination with second‐line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first‐line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double‐blind, multicentre, phase 3 study. The Lancet Oncology 2015;16(5):499‐508. - PubMed
Tebbutt 2010
-
- Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, Hazel GA, et al. Capecitabine, bevacizumab, and mitomycin in first‐line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. Journal of Clinical Oncology 2010;28(19):3191‐8. - PubMed
TGA 2016
-
- Capecitabine colorectal. [Australian Government Department of Health. Therapeutic Goods Administration]. Available from: https://tga‐search.clients.funnelback.com/s/search.html?query=capecitabine%20colorec... (accessed 12 September 2016).
Tierney 2007
Tovey 2012
-
- Tovey D (Editor). Standards for the reporting of new Cochrane Intervention Reviews. Version 1.1. December 2012. Available from: http://editorial‐unit.cochrane.org/mecir.
Van Cutsem 2006
-
- Cutsem E, Nordlinger B, Adam R, Köhne C‐H, Pozzo C, Poston G, et al. Towards a pan‐European consensus on the treatment of patients with colorectal liver metastases. European Journal of Cancer 2006;42(14):2212‐21. - PubMed
Van Cutsem 2011
-
- Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first‐line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. Journal of Clinical Oncology 2011;29(15):2011‐9. - PubMed
Van Cutsem 2012
-
- Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin‐based regimen. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012;30(28):3499‐506. - PubMed
Van der Geest LGM
-
- Geest LGM, Lam‐Boer J, Koopman M, Verhoef C, Elferink MAG, Wilt JHW. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clinical & Experimental Metastasis 2015;32:457‐65. - PubMed
Weiser 2015
-
- Weiser MR, Zhang Z, Schrag D. Locally advanced rectal cancer: time for precision therapeutics. American Society of Clinical Oncology Education Book 2015;e192‐6:doi: 10.14694/EdBook_AM.2015.35.e192.. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

