Antioxidants for female subfertility
- PMID: 28752910
- PMCID: PMC6483341
- DOI: 10.1002/14651858.CD007807.pub3
Antioxidants for female subfertility
Update in
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article.
Abstract
Background: A couple may be considered to have fertility problems if they have been trying to conceive for over a year with no success. This may affect up to a quarter of all couples planning a child. It is estimated that for 40% to 50% of couples, subfertility may result from factors affecting women. Antioxidants are thought to reduce the oxidative stress brought on by these conditions. Currently, limited evidence suggests that antioxidants improve fertility, and trials have explored this area with varied results. This review assesses the evidence for the effectiveness of different antioxidants in female subfertility.
Objectives: To determine whether supplementary oral antioxidants compared with placebo, no treatment/standard treatment or another antioxidant improve fertility outcomes for subfertile women.
Search methods: We searched the following databases (from their inception to September 2016) with no language or date restriction: Cochrane Gynaecology and Fertility Group (CGFG) specialised register, the Cochrane Central Register of Studies (CENTRAL CRSO), MEDLINE, Embase, PsycINFO, CINAHL and AMED. We checked reference lists of appropriate studies and searched for ongoing trials in the clinical trials registers.
Selection criteria: We included randomised controlled trials (RCTs) that compared any type, dose or combination of oral antioxidant supplement with placebo, no treatment or treatment with another antioxidant, among women attending a reproductive clinic. We excluded trials comparing antioxidants with fertility drugs alone and trials that only included fertile women attending a fertility clinic because of male partner infertility.
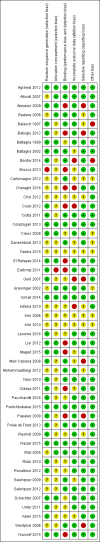
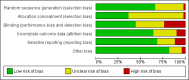
Data collection and analysis: Two review authors independently selected eligible studies, extracted the data and assessed the risk of bias of the included studies. The primary review outcome was live birth; secondary outcomes included clinical pregnancy rates and adverse events. We pooled studies using a fixed-effect model, and calculated odds ratios (ORs) with 95% confidence intervals (CIs) for the dichotomous outcomes of live birth, clinical pregnancy and adverse events. We assessed the overall quality of the evidence by applying GRADE criteria.
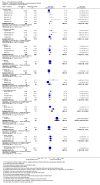
Main results: We included 50 trials involving 6510 women. Investigators compared oral antioxidants, including combinations of antioxidants, N-acetyl-cysteine, melatonin, L-arginine, myo-inositol, D-chiro-inositol, carnitine, selenium, vitamin E, vitamin B complex, vitamin C, vitamin D+calcium, CoQ10, pentoxifylline and omega-3-polyunsaturated fatty acids versus placebo, no treatment/standard treatment or another antioxidant.Very low-quality evidence suggests that antioxidants may be associated with an increased live birth rate compared with placebo or no treatment/standard treatment (OR 2.13, 95% CI 1.45 to 3.12, P > 0.001, 8 RCTs, 651 women, I2 = 47%). This suggests that among subfertile women with an expected live birth rate of 20%, the rate among women using antioxidants would be between 26% and 43%.Very low-quality evidence suggests that antioxidants may be associated with an increased clinical pregnancy rate compared with placebo or no treatment/standard treatment (OR 1.52, 95% CI 1.31 to 1.76, P < 0.001, 26 RCTs, 4271 women, I2 = 66%). This suggests that among subfertile women with an expected clinical pregnancy rate of 22%, the rate among women using antioxidants would be between 27% and 33%. Heterogeneity was moderately high.There was insufficient evidence to determine whether there was a difference between the groups in rates of miscarriage (OR 0.79, 95% CI 0.58 to 1.08, P = 0.14, 18 RCTs, 2834 women, I2 = 23%, very low quality evidence). This suggests that, among subfertile women with an expected miscarriage rate of 7%, use of antioxidants would be expected to result in a miscarriage rate of between 4% and 7%. There was also insufficient evidence to determine whether there was a difference between the groups in rates of multiple pregnancy (OR 1.00, 95% CI 0.73 to 1.38, P = 0.98, 8 RCTs, 2163 women, I2 = 4%, very low quality evidence). This suggests that among subfertile women with an expected multiple pregnancy rate of 8%, use of antioxidants would be expected to result in a multiple pregnancy rate between 6% and 11%. Likewise, there was insufficient evidence to determine whether there was a difference between the groups in rates of gastrointestinal disturbances (OR 1.55, 95% CI 0.47 to 5.10, P = 0.47, 3 RCTs, 343 women, I2 = 0%, very low quality evidence). This suggests that among subfertile women with an expected gastrointestinal disturbance rate of 2%, use of antioxidants would be expected to result in a rate between 1% and 11%. Overall adverse events were reported by 35 trials in the meta-analysis, but there was insufficient evidence to draw any conclusions.Only one trial reported on live birth, clinical pregnancy or adverse effects in the antioxidant versus antioxidant comparison, and no conclusions could be drawn.Very low-quality evidence suggests that pentoxifylline may be associated with an increased clinical pregnancy rate compared with placebo or no treatment (OR 2.07, 95% CI 1.20 to 3.56, P = 0.009, 3 RCTs, 276 women, I2 = 0%). This suggests that among subfertile women with an expected clinical pregnancy rate of 25%, the rate among women using pentoxifylline would be between 28% and 53%.There was insufficient evidence to determine whether there was a difference between the groups in rates of miscarriage (OR 1.34, 95% CI 0.46 to 3.90, P = 0.58, 3 RCTs, 276 women, I2 = 0%) or multiple pregnancy (OR 0.78, 95% CI 0.20 to 3.09, one RCT, 112 women, very low quality evidence). This suggests that among subfertile women with an expected miscarriage rate of 4%, the rate among women using pentoxifylline would be between 2% and 15%. For multiple pregnancy, the data suggest that among subfertile women with an expected multiple pregnancy rate of 9%, the rate among women using pentoxifylline would be between 2% and 23%.The overall quality of evidence was limited by serious risk of bias associated with poor reporting of methods, imprecision and inconsistency.
Authors' conclusions: In this review, there was very low-quality evidence to show that taking an antioxidant may provide benefit for subfertile women, but insufficient evidence to draw any conclusions about adverse events. At this time, there is limited evidence in support of supplemental oral antioxidants for subfertile women.
Conflict of interest statement
Roger Hart is the Medical Director of Fertility Specialists of WA and a shareholder in Western IVF. He has received educational sponsorship from Merck Serono and Ferring pharmaceuticals, and is on the medical advisory board of MSD and Ferring Pharmaceuticals.
Rebecca Mackenzie‐Proctor: no conflict of interest to declare
Vanessa Jordan: no conflict of interest to declare
Marian Showell: no conflict of interest to declare
Figures


























Update of
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2013 Aug 5;(8):CD007807. doi: 10.1002/14651858.CD007807.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Jul 28;7:CD007807. doi: 10.1002/14651858.CD007807.pub3. PMID: 23913583 Updated.
References
References to studies included in this review
-
- Agrawal R, Burt E, Gallagher AM, Butler L, Venkatakrishnan R, Peitsidis P. Prospective randomized trial of multiple micronutrients in subfertile women undergoing ovulation induction: a pilot study. Reproductive Biomedicine Online 2012;24(1):54‐60. - PubMed
-
- Alborzi S, Ghotbi S, Parsanezhad ME, Dehbashi S, Alborzi S, Alborzi M. Pentoxifylline therapy after laparoscopic surgery for different stages of endometriosis: a prospective, double‐blind, randomized, placebo‐controlled study. Journal of Minimally Invasive Gynecology 2007;14(1):54‐8. - PubMed
- Ghotbi S, Parsanezhad ME, Dehbashi S, Alborzi S, Alborzi M. Pentoxifylline therapy after laparoscopic surgery for different stages of endometriosis: A prospective, double‐blind, randomized, placebo‐controlled study. Journal of Minimally Invasive Gynecology2007; Vol. 14, issue 1:54‐8. - PubMed
-
- Aleyasin A, Aghahosseini M, Mohseni M, Mahavi A. Effects of pentoxifylline and vitamin E on pregnancy rate in infertile women by ZIFT: a randomized clinical trial. Iranian Journal of Reproductive Medicine 2009;7:175‐9.
-
- Badawy A, Nashar AB, Totongy M. Clomiphene citrate plus N‐acetyl cysteine versus clomiphene citrate for augmenting ovulation in the management of unexplained infertility: a randomized double‐blind controlled trial. Fertility and Sterility 2006;86(3):647‐50. - PubMed
-
- Balasch J, Creus M, Fabregues F, Carmona F, Martinez‐Roman S, Manau D, et al. Pentoxifylline versus placebo in the treatment of infertility associated with minimal or mild endometriosis. Human Reproduction 1997;12(9):2046‐50. - PubMed
References to studies excluded from this review
-
- Aksoy AN, Kadanali S. Evaluating the effects of vitamin E addition to clomiphene citrate on endometrial receptivity: a prospective controlled study. Turkiye Klinikleri Jinekoloji Obstetrik 2010;20(2):71‐6.
-
- Al‐Omari W, Abdul‐Qadder A, Sulaiman W, Taher M. Antioxidants improve metabolic and ovarian response in polycsytic ovary syndrome. ESHRE. 2003; Vol. xviii:84.
-
- Ardabili HR, Gargari BP, Farzadi L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Nutrition Research 2012;32(3):195‐201. - PubMed
-
- Asadi M, Matin N, Frootan M, Mohamadpour J, Qorbani M, Tanha FD. Vitamin D improves endometrial thickness in PCOS women who need intrauterine insemination: A randomized double‐blind placebo‐controlled trial. Archives of Gynecology and Obstetrics 2014;289(4):865‐870. - PubMed
References to ongoing studies
-
- CTRI/2012/08/002943. Nutritional supplement for women with polycystic ovary syndrome or subfertility. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=5002&EncHid=&... 31st August 2012.
-
- IRCT201112148408N1. Evaluation of the effects of Calcium‐ Vitamine D supplementary on ovulation and fertility outcomes of patients with poly cystic ovary syndrome referring to Infertility Clinic of Imam Khomeini Hospital in 2011 and 2012 for in vitro fertilization. Iranian Registry of Clinical Trials2012.
-
- NCT01019785. Vitamin D during in vitro fertilisation (IVF) ‐ a prospective randomised trial (delivery). clinicaltrials.gov/show/NCT01019785 23 November 2009. [ClinicalTrials.gov : NCT01019785]
-
- NCT01267604. Improving oocyte retrieval using a combined therapy of recombinant follicle stimulating hormone (rFSH) and inositol and melatonin. clinicaltrials.gov/ct2/show/NCT01267604 23rd December 2010. [NCT01267604]
Additional references
-
- Badawy A, State O, Abdelgawad S. N‐acetyl cysteine and clomiphene citrate for induction of ovulation in polycystic ovary syndrome: a cross‐over trial. Acta Obstetricia et Gynecologica Scandinavica 2007;86(2):218‐22. - PubMed
-
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment‐seeking: potential need and demand for infertility medical care. Human Reproduction (Oxford, England) 2007;22(6):1506‐12. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

