Identification of Phosphorylation Codes for Arrestin Recruitment by G Protein-Coupled Receptors
- PMID: 28753425
- PMCID: PMC5567868
- DOI: 10.1016/j.cell.2017.07.002
Identification of Phosphorylation Codes for Arrestin Recruitment by G Protein-Coupled Receptors
Abstract
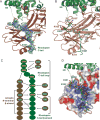
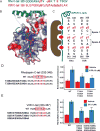
G protein-coupled receptors (GPCRs) mediate diverse signaling in part through interaction with arrestins, whose binding promotes receptor internalization and signaling through G protein-independent pathways. High-affinity arrestin binding requires receptor phosphorylation, often at the receptor's C-terminal tail. Here, we report an X-ray free electron laser (XFEL) crystal structure of the rhodopsin-arrestin complex, in which the phosphorylated C terminus of rhodopsin forms an extended intermolecular β sheet with the N-terminal β strands of arrestin. Phosphorylation was detected at rhodopsin C-terminal tail residues T336 and S338. These two phospho-residues, together with E341, form an extensive network of electrostatic interactions with three positively charged pockets in arrestin in a mode that resembles binding of the phosphorylated vasopressin-2 receptor tail to β-arrestin-1. Based on these observations, we derived and validated a set of phosphorylation codes that serve as a common mechanism for phosphorylation-dependent recruitment of arrestins by GPCRs.
Keywords: GPCR; GRK; arrestin; biased signaling; drug discovery; membrane proteins; phosphorylation codes; rhodopsin.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
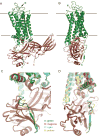




References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

