A Genetic Variant Associated with Five Vascular Diseases Is a Distal Regulator of Endothelin-1 Gene Expression
- PMID: 28753427
- PMCID: PMC5785707
- DOI: 10.1016/j.cell.2017.06.049
A Genetic Variant Associated with Five Vascular Diseases Is a Distal Regulator of Endothelin-1 Gene Expression
Abstract
Genome-wide association studies (GWASs) implicate the PHACTR1 locus (6p24) in risk for five vascular diseases, including coronary artery disease, migraine headache, cervical artery dissection, fibromuscular dysplasia, and hypertension. Through genetic fine mapping, we prioritized rs9349379, a common SNP in the third intron of the PHACTR1 gene, as the putative causal variant. Epigenomic data from human tissue revealed an enhancer signature at rs9349379 exclusively in aorta, suggesting a regulatory function for this SNP in the vasculature. CRISPR-edited stem cell-derived endothelial cells demonstrate rs9349379 regulates expression of endothelin 1 (EDN1), a gene located 600 kb upstream of PHACTR1. The known physiologic effects of EDN1 on the vasculature may explain the pattern of risk for the five associated diseases. Overall, these data illustrate the integration of genetic, phenotypic, and epigenetic analysis to identify the biologic mechanism by which a common, non-coding variant can distally regulate a gene and contribute to the pathogenesis of multiple vascular diseases.
Keywords: SNP; cardiovascular diseases; coronary artery disease; endothelial cells; endothelin-1; epigenomics; genetic enhancer elements; genome-wide association study; hypertension; migraine disorders.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


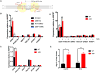


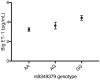

Comment in
-
Complex disease: From non-coding risk variant to biological mechanism in CAD.Nat Rev Genet. 2017 Sep;18(9):514-515. doi: 10.1038/nrg.2017.66. Epub 2017 Aug 7. Nat Rev Genet. 2017. PMID: 28781363 No abstract available.
References
-
- Aguet F, Brown AA, Castel S, Davis JR, Mohammadi P, Segre AV, Zappala Z, Abell NS, Fresard L, Gamazon ER, et al. Local genetic effects on gene expression across 44 human tissues. bioRxiv. 2016 http://dx.doi.org/10.1101/074450. - DOI
-
- Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TL, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110:2233–2240. - PubMed
-
- Anttila V, Winsvold BS, Gormley P, Kurth T, Bettella F, McMahon G, Kallela M, Malik R, de Vries B, Terwindt G, et al. North American Brain Expression Consortium; UK Brain Expression Consortium; International Headache Genetics Consortium. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45:912–917. - PMC - PubMed
-
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning Pv, Larsson HBW, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–645. - PubMed
-
- Bacon CR, Cary NRB, Davenport AP. Endothelin peptide and receptors in human atherosclerotic coronary artery and aorta. Circ Res. 1996;79:794–801. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

