Regeneration of the digestive system in the crinoid Himerometra robustipinna occurs by transdifferentiation of neurosecretory-like cells
- PMID: 28753616
- PMCID: PMC5533335
- DOI: 10.1371/journal.pone.0182001
Regeneration of the digestive system in the crinoid Himerometra robustipinna occurs by transdifferentiation of neurosecretory-like cells
Abstract
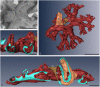
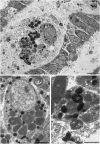
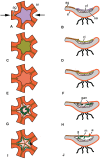
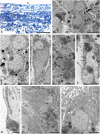
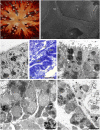
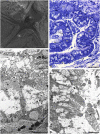
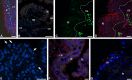
The structure and regeneration of the digestive system in the crinoid Himerometra robustipinna (Carpenter, 1881) were studied. The gut comprises a spiral tube forming radial lateral processes, which gives it a five-lobed shape. The digestive tube consists of three segments: esophagus, intestine, and rectum. The epithelia of these segments have different cell compositions. Regeneration of the gut after autotomy of the visceral mass progresses very rapidly. Within 6 h after autotomy, an aggregation consisting of amoebocytes, coelomic epithelial cells and juxtaligamental cells (neurosecretory neurons) forms on the inner surface of the skeletal calyx. At 12 h post-autotomy, transdifferentiation of the juxtaligamental cells starts. At 24 h post-autotomy these cells undergo a mesenchymal-epithelial-like transition, resulting in the formation of the luminal epithelium of the gut. Specialization of the intestinal epithelial cells begins on day 2 post-autotomy. At this stage animals acquire the mouth and anal opening. On day 4 post-autotomy the height of both the enterocytes and the visceral mass gradually increases. Proliferation does not play any noticeable role in gut regeneration. The immersion of animals in a 10-7 M solution of colchicine neither stopped formation of the lost structures nor caused accumulation of mitoses in tissues. Weakly EdU-labeled nuclei were observed in the gut only on day 2 post-autotomy and were not detected at later regeneration stages. Single mitotically dividing cells were recorded during the same period. It is concluded that juxtaligamental cells play a major role in gut regeneration in H. robustipinna. The main mechanisms of morphogenesis are cell migration and transdifferentiation.
Conflict of interest statement
Figures




References
-
- Becker SF, Jarriault S (2016) Natural and induced direct reprogramming: mechanisms, concepts and general principles—from the worm to vertebrates. Current Opinion in Genetics & Development 40: 154–163. - PubMed
-
- Eguchi G, Kodama R (1993) Transdifferentiation. Current Opinion in Cell Biology 5: 1023–1028. - PubMed
-
- Eguizabal C, Montserrat N, Veiga A, Belmonte JCI (2013) Dedifferentiation, transdifferentiation, and reprogramming: future irections in regenerative medicine. Seminars in Reproductive Medicine 31: 082–094. - PubMed
-
- Fu L, Zhu X, Yi F, Liu G-H, Belmonte JCI (2014) Regenerative medicine: transdifferentiation in vivo. Cell Research 24: 141–142. doi: 10.1038/cr.2013.165 - DOI - PMC - PubMed
-
- Hu CP, Wu XR, Li QG, Sun ZW, Wang AP, Feng JT, et al. (2013) Proteomic analysis of NGF-induced transdifferentiation of adrenal medullary cells. International Journal of Molecular Medicine 32: 347–354. doi: 10.3892/ijmm.2013.1387 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

