Mechanical versus humoral determinants of brain death-induced lung injury
- PMID: 28753621
- PMCID: PMC5533440
- DOI: 10.1371/journal.pone.0181899
Mechanical versus humoral determinants of brain death-induced lung injury
Abstract
Background: The mechanisms of brain death (BD)-induced lung injury remain incompletely understood, as uncertainties persist about time-course and relative importance of mechanical and humoral perturbations.
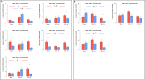
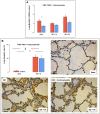
Methods: Brain death was induced by slow intracranial blood infusion in anesthetized pigs after randomization to placebo (n = 11) or to methylprednisolone (n = 8) to inhibit the expression of pro-inflammatory mediators. Pulmonary artery pressure (PAP), wedged PAP (PAWP), pulmonary vascular resistance (PVR) and effective pulmonary capillary pressure (PCP) were measured 1 and 5 hours after Cushing reflex. Lung tissue was sampled to determine gene expressions of cytokines and oxidative stress molecules, and pathologically score lung injury.
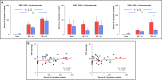
Results: Intracranial hypertension caused a transient increase in blood pressure followed, after brain death was diagnosed, by persistent increases in PAP, PCP and the venous component of PVR, while PAWP did not change. Arterial PO2/fraction of inspired O2 (PaO2/FiO2) decreased. Brain death was associated with an accumulation of neutrophils and an increased apoptotic rate in lung tissue together with increased pro-inflammatory interleukin (IL)-6/IL-10 ratio and increased heme oxygenase(HO)-1 and hypoxia inducible factor(HIF)-1 alpha expression. Blood expressions of IL-6 and IL-1β were also increased. Methylprednisolone pre-treatment was associated with a blunting of increased PCP and PVR venous component, which returned to baseline 5 hours after BD, and partially corrected lung tissue biological perturbations. PaO2/FiO2 was inversely correlated to PCP and lung injury score.
Conclusions: Brain death-induced lung injury may be best explained by an initial excessive increase in pulmonary capillary pressure with increased pulmonary venous resistance, and was associated with lung activation of inflammatory apoptotic processes which were partially prevented by methylprednisolone.
Conflict of interest statement
Figures







References
-
- Davison DL, Terek M and Chawla LS. Neurogenic pulmonary edema. Crit Care 2012;16: 212 doi: 10.1186/cc11226 - DOI - PMC - PubMed
-
- Fein IA and, Rackow EC. Neurogenic pulmonary edema. Chest 1982;81: 318–320. - PubMed
-
- Theodore J and Robin ED. Speculations on neurogenic pulmonary edema (NPE). Am Rev Respir Dis 1976;113: 405–411. doi: 10.1164/arrd.1976.113.4.405 - DOI - PubMed
-
- Demling R, Riessen R. Pulmonary dysfunction after cerebral injury. Crit Care Med. 1990;18: 768–74. - PubMed
-
- Connor RC. Myocardial damage secondary to brain lesions. Am Heart J. 1969;78: 145–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

