Mycobacterium tuberculosis universal stress protein Rv2623 interacts with the putative ATP binding cassette (ABC) transporter Rv1747 to regulate mycobacterial growth
- PMID: 28753640
- PMCID: PMC5549992
- DOI: 10.1371/journal.ppat.1006515
Mycobacterium tuberculosis universal stress protein Rv2623 interacts with the putative ATP binding cassette (ABC) transporter Rv1747 to regulate mycobacterial growth
Abstract
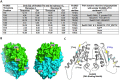
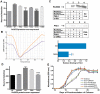
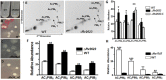
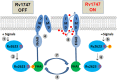
We have previously shown that the Mycobacterium tuberculosis universal stress protein Rv2623 regulates mycobacterial growth and may be required for the establishment of tuberculous persistence. Here, yeast two-hybrid and affinity chromatography experiments have demonstrated that Rv2623 interacts with one of the two forkhead-associated domains (FHA I) of Rv1747, a putative ATP-binding cassette transporter annotated to export lipooligosaccharides. FHA domains are signaling protein modules that mediate protein-protein interactions to modulate a wide variety of biological processes via binding to conserved phosphorylated threonine (pT)-containing oligopeptides of the interactors. Biochemical, immunochemical and mass spectrometric studies have shown that Rv2623 harbors pT and specifically identified threonine 237 as a phosphorylated residue. Relative to wild-type Rv2623 (Rv2623WT), a mutant protein in which T237 has been replaced with a non-phosphorylatable alanine (Rv2623T237A) exhibits decreased interaction with the Rv1747 FHA I domain and diminished growth-regulatory capacity. Interestingly, compared to WT bacilli, an M. tuberculosis Rv2623 null mutant (ΔRv2623) displays enhanced expression of phosphatidyl-myo-inositol mannosides (PIMs), while the ΔRv1747 mutant expresses decreased levels of PIMs. Animal studies have previously shown that ΔRv2623 is hypervirulent, while ΔRv1747 is growth-attenuated. Collectively, these data have provided evidence that Rv2623 interacts with Rv1747 to regulate mycobacterial growth; and this interaction is mediated via the recognition of the conserved Rv2623 pT237-containing FHA-binding motif by the Rv1747 FHA I domain. The divergent aberrant PIM profiles and the opposing in vivo growth phenotypes of ΔRv2623 and ΔRv1747, together with the annotated lipooligosaccharide exporter function of Rv1747, suggest that Rv2623 interacts with Rv1747 to modulate mycobacterial growth by negatively regulating the activity of Rv1747; and that Rv1747 might function as a transporter of PIMs. Because these glycolipids are major mycobacterial cell envelope components that can impact on the immune response, our findings raise the possibility that Rv2623 may regulate bacterial growth, virulence, and entry into persistence, at least in part, by modulating the levels of bacillary PIM expression, perhaps through negatively regulating the Rv1747-dependent export of the immunomodulatory PIMs to alter host-pathogen interaction, thereby influencing the fate of M. tuberculosis in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Flynn JL, Chan J (2001) Immunology of tuberculosis. Annu Rev Immunol 19: 93–129. doi: 10.1146/annurev.immunol.19.1.93 - DOI - PubMed
-
- Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, et al. (2009) The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 7: 845–855. doi: 10.1038/nrmicro2236 - DOI - PMC - PubMed
-
- Chan J, Flynn J (2004) The immunological aspects of latency in tuberculosis. Clin Immunol 110: 2–12. - PubMed
-
- Gomez JE, McKinney JD (2004) M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 84: 29–44. - PubMed
MeSH terms
Substances
Grants and funding
- R01 AI098925/AI/NIAID NIH HHS/United States
- R37 AI026170/AI/NIAID NIH HHS/United States
- T32 AI007501/AI/NIAID NIH HHS/United States
- R01 AI093570/AI/NIAID NIH HHS/United States
- R01 AI026170/AI/NIAID NIH HHS/United States
- U54 GM094662/GM/NIGMS NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- U54 GM093342/GM/NIGMS NIH HHS/United States
- R01 GM115773/GM/NIGMS NIH HHS/United States
- R01 AI093649/AI/NIAID NIH HHS/United States
- P01 GM118303/GM/NIGMS NIH HHS/United States
- R01 GM086482/GM/NIGMS NIH HHS/United States
- S10 RR031795/RR/NCRR NIH HHS/United States
- P01 AI063537/AI/NIAID NIH HHS/United States
- R01 AI094745/AI/NIAID NIH HHS/United States
- R01 GM126297/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

