Bacterial effector NleL promotes enterohemorrhagic E. coli-induced attaching and effacing lesions by ubiquitylating and inactivating JNK
- PMID: 28753655
- PMCID: PMC5549993
- DOI: 10.1371/journal.ppat.1006534
Bacterial effector NleL promotes enterohemorrhagic E. coli-induced attaching and effacing lesions by ubiquitylating and inactivating JNK
Abstract
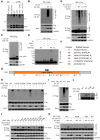
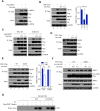
As a major diarrheagenic human pathogen, enterohemorrhagic Escherichia coli (EHEC) produce attaching and effacing (A/E) lesions, characterized by the formation of actin pedestals, on mammalian cells. A bacterial T3SS effector NleL from EHEC O157:H7 was recently shown to be a HECT-like E3 ligase in vitro, but its biological functions and host targets remain elusive. Here, we report that NleL is required to effectively promote EHEC-induced A/E lesions and bacterial infection. Furthermore, human c-Jun NH2-terminal kinases (JNKs) were identified as primary substrates of NleL. NleL-induced JNK ubiquitylation, particularly mono-ubiquitylation at the Lys 68 residue of JNK, impairs JNK's interaction with an upstream kinase MKK7, thus disrupting JNK phosphorylation and activation. This subsequently suppresses the transcriptional activity of activator protein-1 (AP-1), which modulates the formation of the EHEC-induced actin pedestals. Moreover, JNK knockdown or inhibition in host cells complements NleL deficiency in EHEC infection. Thus, we demonstrate that the effector protein NleL enhances the ability of EHEC to infect host cells by targeting host JNK, and elucidate an inhibitory role of ubiquitylation in regulating JNK phosphorylation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
The EHEC type III effector NleL is an E3 ubiquitin ligase that modulates pedestal formation.PLoS One. 2011 Apr 26;6(4):e19331. doi: 10.1371/journal.pone.0019331. PLoS One. 2011. PMID: 21541301 Free PMC article.
-
The Canonical Long-Chain Fatty Acid Sensing Machinery Processes Arachidonic Acid To Inhibit Virulence in Enterohemorrhagic Escherichia coli.mBio. 2021 Jan 19;12(1):e03247-20. doi: 10.1128/mBio.03247-20. mBio. 2021. PMID: 33468701 Free PMC article.
-
Interaction of enterohemorrhagic Escherichia coli (EHEC) with mammalian cells: cell adhesion, type III secretion, and actin pedestal formation.Curr Protoc Microbiol. 2007 Jun;Chapter 5:Unit 5A.1. doi: 10.1002/9780471729259.mc05a01s05. Curr Protoc Microbiol. 2007. PMID: 18770622
-
Enterohemorrhagic Escherichia coli Adhesins.Microbiol Spectr. 2014 Jun;2(3). doi: 10.1128/microbiolspec.EHEC-0003-2013. Microbiol Spectr. 2014. PMID: 26103974 Review.
-
Escherichia coli type III secretion system 2: a new kind of T3SS?Vet Res. 2014 Mar 19;45(1):32. doi: 10.1186/1297-9716-45-32. Vet Res. 2014. PMID: 24641581 Free PMC article. Review.
Cited by
-
The NEL Family of Bacterial E3 Ubiquitin Ligases.Int J Mol Sci. 2022 Jul 13;23(14):7725. doi: 10.3390/ijms23147725. Int J Mol Sci. 2022. PMID: 35887072 Free PMC article. Review.
-
Ubiquitin-targeted bacterial effectors: rule breakers of the ubiquitin system.EMBO J. 2023 Sep 18;42(18):e114318. doi: 10.15252/embj.2023114318. Epub 2023 Aug 9. EMBO J. 2023. PMID: 37555693 Free PMC article. Review.
-
Bacterial ligases reveal fundamental principles of polyubiquitin specificity.Mol Cell. 2023 Dec 21;83(24):4538-4554.e4. doi: 10.1016/j.molcel.2023.11.017. Epub 2023 Dec 12. Mol Cell. 2023. PMID: 38091999 Free PMC article.
-
Divergence of Legionella Effectors Reversing Conventional and Unconventional Ubiquitination.Front Cell Infect Microbiol. 2020 Aug 21;10:448. doi: 10.3389/fcimb.2020.00448. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32974222 Free PMC article. Review.
-
Enterohemorrhagic E. coli effector NleL disrupts host NF-κB signaling by targeting multiple host proteins.J Mol Cell Biol. 2020 May 18;12(4):318-321. doi: 10.1093/jmcb/mjaa003. J Mol Cell Biol. 2020. PMID: 32065237 Free PMC article. No abstract available.
References
-
- Gallegos KM, Conrady DG, Karve SS, Gunasekera TS, Herr AB, Weiss AA. Shiga toxin binding to glycolipids and glycans. PLoS one. 2012;7(2):e30368 doi: 10.1371/journal.pone.0030368 - DOI - PMC - PubMed
-
- Campellone KG, Leong JM. Tails of two Tirs: actin pedestal formation by enteropathogenic E-coli and enterohemorrhagic E-coli O157:H7. Curr Opin Microbiol. 2003;6(1):82–90. - PubMed
-
- Goosney DL, DeVinney R, Finlay BB. Recruitment of cytoskeletal and signaling proteins to enteropathogenic and enterohemorrhagic Escherichia coli pedestals. Infect Immun. 2001;69(5):3315–22. doi: 10.1128/IAI.69.5.3315-3322.2001 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

