Overexpression of Arabidopsis FLOWERING LOCUS T (FT) gene improves floral development in cassava (Manihot esculenta, Crantz)
- PMID: 28753668
- PMCID: PMC5533431
- DOI: 10.1371/journal.pone.0181460
Overexpression of Arabidopsis FLOWERING LOCUS T (FT) gene improves floral development in cassava (Manihot esculenta, Crantz)
Abstract
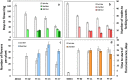
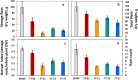
Cassava is a tropical storage-root crop that serves as a worldwide source of staple food for over 800 million people. Flowering is one of the most important breeding challenges in cassava because in most lines flowering is late and non-synchronized, and flower production is sparse. The FLOWERING LOCUS T (FT) gene is pivotal for floral induction in all examined angiosperms. The objective of the current work was to determine the potential roles of the FT signaling system in cassava. The Arabidopsis thaliana FT gene (atFT) was transformed into the cassava cultivar 60444 through Agrobacterium-mediated transformation and was found to be overexpressed constitutively. FT overexpression hastened flower initiation and associated fork-type branching, indicating that cassava has the necessary signaling factors to interact with and respond to the atFT gene product. In addition, overexpression stimulated lateral branching, increased the prolificacy of flower production and extended the longevity of flower development. While FT homologs in some plant species stimulate development of vegetative storage organs, atFT inhibited storage-root development and decreased root harvest index in cassava. These findings collectively contribute to our understanding of flower development in cassava and have the potential for applications in breeding.
Conflict of interest statement
Figures
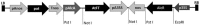



References
-
- Ceballos H, Pérez JC, Joaqui Barandica O, Lenis JI, Morante N, Calle F, et al. Cassava breeding I: The value of breeding value. Frontiers in Plant Science. 2016;7(1227). doi: 10.3389/fpls.2016.01227 - DOI - PMC - PubMed
-
- Ceballos H, Iglesias CA, Perez JC, Dixon AGO. Cassava breeding: opportunities and challenges. Plant Molecular Biology. 2004;56(4):503–16. doi: 10.1007/s11103-004-5010-5 - DOI - PubMed
-
- Ceballos H, Kawuki RS, Gracen VE, Yencho GC, Hershey CH. Conventional breeding, marker-assisted selection, genomic selection and inbreeding in clonally propagated crops: a case study for cassava. Theoretical and Applied Genetics. 2015;128(9):1647–67. doi: 10.1007/s00122-015-2555-4 - DOI - PMC - PubMed
-
- Turck F, Fornara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annual Review of Plant Biology. 2008;59:573–94. doi: 10.1146/annurev.arplant.59.032607.092755 - DOI - PubMed
-
- Shalit A, Rozman A, Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y, et al. The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proceedings of the National Academy of Sciences. 2009;106(20):8392–7. doi: 10.1073/pnas.0810810106 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

