Predictive factors and prevalence of microalbuminuria in HIV-infected patients: a cross-sectional analysis
- PMID: 28754089
- PMCID: PMC5534061
- DOI: 10.1186/s12882-017-0672-9
Predictive factors and prevalence of microalbuminuria in HIV-infected patients: a cross-sectional analysis
Abstract
Background: Renal dysfunction is a common problem in the HIV+ population, due to the effect of both the HIV virus and the several classes of ARV drugs such as tenofovir (TDF). It is also known that the presence of renal damage correlates with cardiovascular risk and therefore with the risk of mortality of the patients accordingly. The detection of early renal damage is very important. Albuminuria and microalbuminuria are markers of early kidney disease and cardiovascular risk. The aim of the study is to evaluate the prevalence of microalbuminuria in a large polycentric sample, of unselected and consecutive HIV-patients followed as outpatients, and to assess its association with different therapeutic regimens.
Methods: We studied 326 patients with a mean age of 48.4 ± 1.6 years, treated at the Infectious Diseases Clinics of Chieti and Perugia for 48 weeks. The main metabolic parameters and the microalbuminuria levels in a single sample of urine were evaluated.
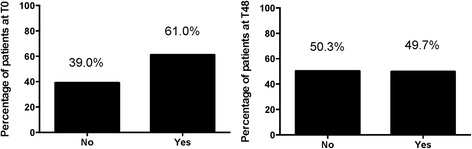
Results: Microalbuminuria was detected in 61.0% of patients at T0 and in 49.7% after 48 weeks of observation with a median values of 1.1 mg/L (IQR: 0-2.7) vs. 0 mg/L (IQR: 0-2.0). 70% of the enrolled population did not show changes in microalbuminuria levels over time, 19% showed improvement, and 11% of the population had a worsening of microalbuminuria levels without any alteration of creatinine, uric acid and GFR-MDRD. We also found a statistically significant association between the development of microalbuminuria and gender (p < 0.035), Arterial Hypertension (AH) (p < 0.028) and therapy with TDF (p < 0.050).
Conclusion: We showed a very high prevalence of microalbuminuria, much higher than the literature data; the use of TDF affects the renal function in a statistically significant way and should therefore be considered a risk factor for kidney damage, which can be early assessed with the measurement of microalbuminuria.
Keywords: Adverse events; Antiretroviral therapy; Kidneys.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee at the University “G. d’Annunzio” Chieti-Pescara (Ethics Committee Project No.19 the 18/12/2014) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The participants provided written informed consent to participate in this study.
Consent for publication
Not applicable since there aren’t any details, images, or videos relating to individual participants included in this article.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, El-Sadr W, Fontas E, Worm S, Kirk O, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491–501. doi: 10.1097/HJR.0b013e328336a150. - DOI - PubMed
-
- Friis-Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d'Arminio Monforte A, Pradier C, Morfeldt L, Mateu S, Law M, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17(8):1179–1193. doi: 10.1097/00002030-200305230-00010. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

